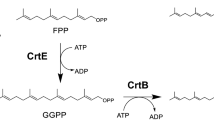
Abstract
Norisoprenoids are produced from carotenoids under oxidative degradation mediated by carotenoid cleavage dioxygenases (CCDs) and contribute to floral and fruity notes in grape berries and wine. The diversity of CCD substrates and products has been demonstrated by in vitro recombinant proteins extracted from Escherichia coli expressing CCD genes and of in vivo proteins in an E. coli system co-expressing genes for carotenoid synthesis and cleavage. In the current study, VvCCD1 and VvCCD4b were isolated from the cDNA library of Vitis vinifera L. cv. Cabernet Sauvignon and then transformed into carotenoid-accumulating recombinant Saccharomyces cerevisiae strains. The expression of the target genes was monitored during the yeast growth period, and the accumulation of carotenoids and norisoprenoids in the recombinant strains was measured. The results indicated that both of the VvCCDs cleaved β-carotene at the 7, 8 (7′, 8′) position into β-cyclocitral for the first time. Additionally, the two enzymes also degraded β-carotene at the 9, 10 (9′, 10′) position to generate β-ionone and cleaved lycopene at the 5, 6 (5′, 6′) position into 6-methyl-5-hepten-2-one. These findings suggested that the VvCCDs may possess more cleavage characteristics under the eukaryotic expression system in S. cerevisiae than the prokaryotic system in E. coli, which could better explain the biochemical functions of VvCCDs in grape berries.






Similar content being viewed by others
References
Sabon I, Revel GD, Yorgos Kotseridis A, Bertrand A (2002) Determination of volatile compounds in grenache wines in relation with different terroirs in the rhone valley. J Agric Food Chem 50:6341–6345
Kotseridis Y, Baumes RL, Bertrand A, Skouroumounis GK (1999) Quantitative determination of β-ionone in red wines and grapes of bordeaux using a stable isotope dilution assay. J Chromatogr A 848:317–325
Kotseridis Y, Baumes R, Skouroumounis KG (1998) Synthesis of labelled [2H4] β-damascenone, [2H2]2-methoxy-3- isobutylpyrazine, [2H3]α-ionone, and [2H3]β-ionone, for quantification in grapes, juices and wines. J Chromatogr A 824:71–78
Aznar M, López R, Cacho J, Ferreira V (2001) Identification and quantification of impact odorants of aged red wines from Rioja. GC-Olfactometry, quantitative GC-MS, and odor evaluation of hplc fractions. J Agric Food Chem 49:2924–2929
Mateo JJ, Jiménez M (2000) Monoterpenes in grape juice and wines. J Chromatogr A 881:557–567
Ristic R, Bindon K, Francis LI, Herderich MJ, Iland PG (2010) Flavonoids and C13-norisoprenoids in Vitis vinifera L. cv. Shiraz: relationships between grape and wine composition, wine colour and wine sensory properties. Aust J Grape Wine R 16:369–388
Wang XJ, Tao YS, Wu Y, An RY, Yue ZY (2017) Aroma compounds and characteristics of noble-rot wines of chardonnay grapes artificially botrytized in the vineyard. Food Chem 226:41–50
Schwartz SH (1997) Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276:1872–1874
Tan BC, Joseph LM, Deng WT, Liu L, Li QB, Cline K, McCarty RD (2003) Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J 35(1):44–56
Schwartz SH, Qin X, Zeevaart JA (2001) Characterization of a novel carotenoid cleavage dioxygenase from plants. J Biol Chem 276:25208–25211
Luchi S, Kobayashi M, Taji T, Naramoto M, Seki M, Kato T, Tabata S, Kakubari Y, Yamaguchi-Shinozaki K, Shinozaki K (2001) Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase: a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J 27:325–333
Schwartz SH, Qin X, Loewen MC (2004) The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J Biol Chem 279:46940–46945
Bouvier F (2003) Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis. Plant Cell 15:47–62
Frusciante S, Diretto G, Bruno M, Ferrante P, Pietrella M, Prado-Cabrero A, Rubio-Moraga A, Beyer P, Gomez-Gomez L, Al-Babili S, Giuliano G (2014) Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc Natl Acad Sci USA 111:12246–12251
Ahrazem O, Rubiomoraga A, Nebauer SG, Molina RV, Gómezgómez L (2015) Saffron: its phytochemistry, developmental processes, and biotechnological prospects. J Agric Food Chem 63:8751–8764
Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ (2004) The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone, and geranylacetone. Plant J 40:882–892
Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya K, Schmelz E, Clark GD, Klee JH (2004) Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of β-ionone, a fragrance volatile of petunia flowers. Plant Physiol 136:3504–3514
Baldermann S, Naim M, Fleischmann P (2005) Enzymatic carotenoid degradation and aroma formation in nectarines (Prunus persica). Food Res Int 38:833–836
Garcia-Limones C, Schnabele K, Blanco-Portales R, Bellido ML, Caballero JL, Schwab W, Muñoz-Blanco J (2008) Functional characterization of FaCCD1: a carotenoid cleavage dioxygenase from strawberry involved in lutein degradation during fruit ripening. J Agric Food Chem 56:9277–9285
Rodrigo MJ, Alquéz ZB, Alós E, Medina V, Carmona L, Bruno M, Al-Babili S, Zacarías L (2013) A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments. J Exp Bot 64:4461–4478
Lashbrooke JG, Young PR, Dockrall SJ, Vasanth K, Vivier MA (2013) Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family. BMC Plant Biol 13(1):156. https://doi.org/10.1186/1471-2229-13-156
Ibdah M, Azulay Y, Portnoy V, Wasserman B, Bar E, Meir A, Burger Y, Hirschberg J, Schaffer AA, Katzir N, Tadmor Y, Lewinsohn E (2006) Functional characterization of CmCCD1, a carotenoid cleavage dioxygenase from melon. Phytochemistry 67:1579–1589
Zhao SC, Tuan PA, Kim JK, Park WT, Kim YB, Arasu MV, Al-Dhabi NA, Yang JL, Li CH, Park SU (2014) Molecular characterization of carotenoid biosynthetic genes and carotenoid accumulation in Lycium chinense. Molecules 19:11250–11262
Alder A, Holdermann I, Beyer P, Al-Babili S (2008) Carotenoid oxygenases involved in plant branching catalyse a highly specific conserved apocarotenoid cleavage reaction. Biochem J 416:289–296
Mathieu S, Terrier N, Procureur J, Bigey F, Günata Z (2005) A carotenoid cleavage dioxygenase from vitis vinifera L.: functional characterization and expression during grape berry development in relation to C13-norisoprenoid accumulation. J Exp Bot 56:2721–2731
Huang FC, Horváth G, Molnár P, Turcsi E, József D, Schrader J, Sandmann G, Schmidt H, Schwab W (2009) Substrate promiscuity of RdCCD1, a carotenoid cleavage oxygenase from Rosa damascena. Phytochemistry 70:457–464
Sun Z, Hans J, Walter MH, Matusova R, Beekwilder J, Verstappen FWA, Zhao M, Esther VE, Dieter S, Ton B, Harro JB (2008) Cloning and characterisation of a maize carotenoid cleavage dioxygenase (ZmCCD1) and its involvement in the biosynthesis of apocarotenoids with various roles in mutualistic and parasitic interactions. Planta 228(5):789
Vogel JT, Tan BC, McCarty DR, Klee HJ (2008) The carotenoid cleavage dioxygenase 1 enzyme has broad substrate specificity, cleaving multiple carotenoids at two different bond positions. J Biol Chem 283(17):11364–11373
Fleischmann P, Watanabe N, Winterhalter P (2003) Enzymatic carotenoid cleavage in star fruit (Averrhoa carambola). Phytochemistry 63:131–137
Leavitt JM, Tong A, Tong J, Pattie J, Alper HS (2016) Coordinated transcription factor and promoter engineering to establish strong expression elements in Saccharomyces cerevisiae. Biotechnol J 11:866–876
Verwaal R, Wang J, Meijnen JP, Visser H, Sandmann G, van den Berg JA, van Ooyen AJJ (2007) High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl Environ Microbiol 73:4342–4350
Amiri P, Shahpiri A, Asadollahi MA, Momenbeik F, Partow S (2016) Metabolic engineering of Saccharomyces cerevisiae for linalool production. Biotechnol Lett 38:503–508
Oswald M, Fischer M, Dirninger N, Karst F (2007) Monoterpenoid biosynthesis in Saccharomyces cerevisiae. FEMS Yeast Res 7:413–421
Kong JQ, Lu D, Wang ZB (2014) Molecular cloning and yeast expression of cinnamate 4-hydroxylase from Ornithogalum saundersiae baker. Molecules 19:1608–1621
Yamano S, Ishii T, Nakagawa M, Ikenaga H, Misawa N (2014) Metabolic Engineering for production of β-carotene and lycopene in Saccharomyces cerevisiae. Biosci Biotechnol Biochem 58:1112–1114
Bhataya A, Schmidt-Dannert C, Lee PC (2009) Metabolic engineering of Pichia pastoris X-33 for lycopene production. Process Biochem 44:1095–1102
Araya-Garay JM, Feijoo-Siota L, Rosa-dos-Santos F, Veiga-Crespo P, Villa TG (2012) Construction of new Pichia pastoris X-33 strains for production of lycopene and β-carotene. Appl Environ Microbiol 93:2483–2492
Beekwilder J, Van Rossum HM, Koopman F, Sonntag F, Buchhaupt M, Schrader J, Hallabe DR, Boschab D, Pronkbc TJ, van Marisbc JAA, Daranbc J-M (2014) Polycistronic expression of a β-carotene biosynthetic pathway in Saccharomyces cerevisiae coupled to β-ionone production. J Biotechnol 192:383–392
López J, Essus K, Kim IK, Pereira R, Herzog J, Siewers V, Nielsen J, Agosin E (2015) Production of β-ionone by combined expression of carotenogenic and plant CCD1 genes in Saccharomyces cerevisiae. Microb Cell Fact 14(1):84. https://doi.org/10.1186/s12934-015-0273-x
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Rivas R, Vizcaíno N, Buey RM, Mateos PF, Martínez-Molina E, Velázquez E (2001) An effective, rapid and simple method for total RNA extraction from bacteria and yeast. J Microbiol Meth 47:59–63
Sun RZ, Pan QH, Duan CQ, Wang J (2015) Light response and potential interacting proteins of a grape flavonoid 3′-hydroxylase gene promoter. Plant Physiol Biochem 97:70–81
Zhao X, Shi F, Zhan W (2015) Overexpression of ZWF1 and POS5improves carotenoid biosynthesis in recombinant Saccharomyces cerevisiae. Lett Appl Microbiol 61:354–360
Liu PT, Sun L, Sun YX, Shang F, Yan GL (2016) Decreased fluidity of cell membranes causes a metal ion deficiency in recombinant Saccharomyces cerevisiae, producing carotenoids. J Ind Microbiol Biot 43:525–535
Wu Y, Pan Q, Qu W, Duan C (2009) Comparison of volatile profiles of nine litchi (Litchi chinensis Sonn.) cultivars from Southern China. J Agric Food Chem 57:9676–9681
Floss SD, Walter HM (2006) Role of carotenoid cleavage dioxygenase 1 (CCD1) in apocarotenoid biogenesis revisited. Plant Signal Behav 4:172–175
Baldermann S, Kato M, Kurosawa M, Kurobayashi Y, Fujita A, Fleischmann P, Watanabe N (2010) Functional characterization of a carotenoid cleavage dioxygenase 1 and its relation to the carotenoid accumulation and volatile emission during the floral development of Osmanthus fragrans Lour. J Exp Bot 61:2967–2977
Ilg A, Beyer P, Al-Babili S (2009) Characterization of the rice carotenoid cleavage dioxygenase 1 reveals a novel route for geranial biosynthesis. FEBS J 276:736–747
Ilg A, Bruno M, Beyer P, Al-Babili S (2014) Tomato carotenoid cleavage dioxygenases 1A and 1B: relaxed double bond specificity leads to a plenitude of dialdehydes, mono-apocarotenoids and isoprenoid volatiles. FEBS Open Bio 4:584–593
Ohmiya A, Kishimoto S, Aida R, Yoshioka S, Sumitomo K (2006) Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiol 142:1193–1201
Brandi F, Bar E, Mourgues F, Horváth G, Turcsi E, Giuliano G, Liverani A, Tartarin S, Lewinsohn E, Rosati C (2011) Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol 11(1):24. https://doi.org/10.1186/1471-2229-11-24
Hai NTL, Masuda JI, Miyajima I, Thien NQ, Mojtahedi N, Hiramatsu M, Kim JH, Okubo H (2012) Involvement of carotenoid cleavage dioxygenase 4 gene in tepal color change in Lilium brownii var. colchesteri. J Jpn Soc Hortic Sci 81:366–373
Adami M, Franceschi PD, Brandi F, Liverani A, Giovannini D, Rosati C, Dondino L, Tartarini S (2013) Identifying a carotenoid cleavage dioxygenase (ccd4) gene controlling yellow/white fruit flesh color of peach. Plant Mol Biol Rep 31:1166–1175
Winterhalter P, Rouseff R (2001) Carotenoid-derived aroma compounds. American Chemical Society, Washington, DC
Marais J, van Wyk CJ, Rapp A (1991) Carotenoid levels in maturing grapes as affected by climatic regions, sunlight and shade. S Afr J Enol Vitic 12:64–69
Yuan F, Qian MC (2016) Development of C13-norisoprenoids, carotenoids and other volatile compounds in Vitis vinifera L. cv. Pinot noir grapes. Food Chem 192:633–641
Chen WK, Yu KJ, Liu B, Lan YB, Sun RZ, Li Q, He F, Pan QH, Duan CQ, Wang J (2017) Comparison of transcriptional expression patterns of carotenoid metabolism in ‘Cabernet Sauvignon’ grapes from two regions with distinct climate. J Plant Physiol 213:75–86
Young PR, Lashbrooke JG, Alexandersson E, Jacobson D, Moser C, Velasco R, Vivier AM (2012) The genes and enzymes of the carotenoid metabolic pathway in Vitis vinifera L. BMC Genomics 13:243
Bindon KA, Dry PR, Loveys BR (2007) Influence of plant water status on the production of C13-norisoprenoid precursors in Vitis vinifera L. cv. Cabernet Sauvignon grape berries. J Agric Food Chem 55:4493–4500
Pineau B, Barbe JC, Van Leeuwen C, Dubourdieu D (2007) Which impact for β-damascenone on red wines aroma? J Agric Food Chem 55:4103–4108
Marais J (1994) Sauvignon blanc cultivar aroma-a review. S Afr J Enol Vitic 15(2):41–45. https://doi.org/10.21548/15-2-2283
Rubio-Moraga A, Rambla JL, Fernández-de-Carmen A, Trapero-Mozos A, Ahrazem O, Orzaez D, Granell A, Gómez-Gómez L (2014) New target carotenoids for CCD4 enzymes are revealed with the characterization of a novel stress-induced carotenoid cleavage dioxygenase gene from Crocus sativus. Plant Mol Biol 85:555–569
Mendes-Pinto MM (2009) Carotenoid breakdown products the – norisoprenoids - in wine aroma. Arch Biochem Biophys 483:236–245
Funding
This research was funded by National Natural Science Foundation of China, Grant Number 31272118, and the China Agriculture Research System (CARS-29). Qiu-Hong Pan received the funding. The funders had no role in study design, data collection and analysis, preparation of manuscript, and decision on publication.
Author information
Authors and Affiliations
Contributions
Project administration, QHP; Formal analysis and writing - original draft, NM; Writing - review & editing, GLY, CQD and QHP; Methodology, DZ; Data curation, XYL.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Meng, N., Yan, GL., Zhang, D. et al. Characterization of two Vitis vinifera carotenoid cleavage dioxygenases by heterologous expression in Saccharomyces cerevisiae. Mol Biol Rep 46, 6311–6323 (2019). https://doi.org/10.1007/s11033-019-05072-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05072-3