Abstract
Novel α-(1 → 3)-glucooligosaccharides (α-(1 → 3)-GOS) were prepared by acid hydrolysis of α-(1→ 3)-glucan isolated from Fomitopsis betulina fruiting bodies and characterized. Their anti-cancer potential was evaluated in in vitro assays in a colon cancer cell model. The tested α-(1 → 3)-GOS showed antiproliferative (MTT assay) and pro-apoptotic (Annexin V-FITC and PI technique) features against colon cancer but not against normal epithelial colon cells. Additionally, we did not observe cytotoxic activity (neutral red and lactate dehydrogenase assays) of α-(1 → 3)-GOS against several types of normal cell lines. In the present study, we demonstrated the anticancer potential of α-(1 → 3)-GOS in a colon carcinoma model. The anti-tumour effect of α-(1 → 3)-GOS is related with induction of apoptosis. Based on these results, we conclude that α-(1 → 3)-GOS may be considered as a dietary or therapeutic agent with an ability to inhibit the growth of cancer cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oligosaccharides are a diverse group of sugars containing usually up to 20 monomers in the chain. They occur naturally, can be synthesized chemically or biologically by fermentation of other sugars [16], and can be obtained by chemical [18] or enzymatic hydrolysis [9] of various polysaccharides. Many oligosaccharides exhibit biological activity including anticancer, anti-inflammatory, and immunostimulatory properties [9, 16].
The cell wall of mushrooms and fungi is a rich source of valuable polysaccharides. Fomitopsis betulina (Bull. ex Fr.) P. Karst. (formerly Piptoporus betulinus) is an edible (young fruiting bodies) basidiomycete polypore growing on the wood of birch Betula spp. The fruiting bodies of the fungus have been commonly used in the traditional folk medicine in the Baltic Sea area for their therapeutic properties, which are also reflected in the results of modern research on their biological activity [2, 11, 13]. The Fomitopsis cell wall contains glucans: β-glucans commonly associated with anticancer properties [22] and water-insoluble, alkali-soluble α-glucans [3, 7]. The main chain of α-glucan isolated by Wiater et al. [20] contained 84.6% of α-D-glucopyranose units linked with (1 → 3) bonds in addition to (1 → 4)-linked units. Although α-glucans themselves are considered to be biologically inactive, their water-soluble form, i.e. a carboxymethylated derivative, showed moderate cytotoxic effects in vitro [20].
The anticancer activity of α-(1 → 3)-glucooligosaccharides has not been evaluated so far. In our study, we prepared and analysed a hydrolysate of α-(1 → 3)-glucan isolated from the cell wall of F. betulina fruiting bodies and examined the impact of a mixture of α-(1 → 3)-glucooligosaccharides on colon cancer cell lines in in vitro assays.
Materials and methods
Reagents
All reagents were purchased from Sigma-Aldrich (St Louis, Missouri, USA) unless otherwise indicated.
Preparation of α-(1 → 3)-oligosaccharides
Fruiting bodies of F. betulina were obtained from infected Betula pendula Roth. trees in Lublin and its surroundings, Poland. Isolation of the cell wall material from the fruiting bodies and an alkali-soluble α-(1 → 3)-glucan was carried out according to Wiater et al. [20]. Crude oligosaccharides were obtained by partial acid degradation of α-(1 → 3)-glucan (50 g) in 200 ml of 0.1 M H2SO4 for 1 h at 100 °C. The residues were removed by centrifugation (10 min, 12,000 rpm) and the supernatant was neutralized with CaCO3. After re-centrifuging (10 min, 12,000 rpm), the soluble fraction of the hydrolysate was desalted with Amberlite MB3 (Merck, Germany). The desalted solution containing α-(1 → 3)-glucooligosaccharides was concentrated to 20 ml using a rotary evaporator at 40 °C under vacuum and freeze-dried.
Evaluation of α-(1 → 3)-glucan hydrolysis products
The products of α-(1 → 3)-glucan hydrolysis were analysed by MALDI-TOF MS spectrometry performed with a Waters Synapt G2-Si HDMS instrument (Waters Corporation, Milford, MA, USA) equipped with a 1 kHz Nd:YAG laser system [21]. The sample was dissolved in 50% MeOH at a concentration of 20 µg/µl and mixed with a DHB matrix as described by [1].
Cell lines
Human colon adenocarcinoma (HT-29, LS180, SW620, SW948), human colon epithelial CCD 841 CoTr, and mouse fibroblast L929 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, Virginia, USA). Human skin fibroblasts (HSF) were a laboratory line obtained with the outgrowth technique from skin explants taken from young volunteers with informed donor consent. The cells were cultured in Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F12 Ham containing 10% of foetal bovine serum (FBS), 100 units/ml of penicillin, and 100 μg/ml of streptomycin at 37 °C in atmosphere of 95% air and 5% CO2 unless otherwise indicated.
Cell proliferation assessment
HT-29, LS180, SW620, SW948, and CCD 841 CoTr cells were seeded into 96-well flat bottom microplates at a density of 3 × 104 cells/mL. The next day, the culture medium was removed and the cells were exposed to serial dilutions of α-(1 → 3)-GOS (100 μL, final concentration from 1 to 10 mg/mL). After 96 h of incubation, the cells were exposed to MTT reduction assays [17]. Camptothecin was used as a positive control. IC50 (concentration causing proliferation inhibition by 50% compared to the control) was calculated from the nonlinear regression (log(inhibitor) vs. normalized response-variable slope) using GraphPad Prism 5.0.
Cell viability assessment
HT-29, CCD 841 CoTr, L929, and HSF cells were seeded into 96-well flat bottom microplates at a density of 5 × 105 cells/mL. The next day, the cells were exposed to serial dilutions of α-(1 → 3)-GOS at concentrations ranging from 1 to 10 mg/mL, camptothecin, and a mixture of camptothecin and α-(1 → 3)-GOS prepared in medium with a reduced content of serum (2% FBS). After 24 h of incubation, neutral red (NR) and lactate dehydrogenase (LDH) viability assays were applied (according to the manufacturer’s instructions) to verify the cytotoxicity of α-(1 → 3)-GOS against human colon epithelial cells CCD 841 CoTr, human skin fibroblasts (HSF), mouse fibroblasts, and human colon adenocarcinoma cells.
Analysis of apoptosis and necrosis
Cells were seeded into 6-well microplates at a density of 1 × 106 cells/ml. The next day, the cells were treated with α-(1 → 3)-GOS (2 mL, final concentration from 1 to 10 mg/mL with 2% FBS). After 24 h incubation, the cells were harvested, washed in PBS, and centrifuged. The number of apoptotic and necrotic cells was measured with the Annexin V-FITC and PI technique (BD Biosciences, FITC Annexin V Apoptosis Detection Kit I) according to the manufacturer’s instructions, with a flow cytometer (BD FACSCalibur). Camptothecin was used as a positive control.
Results and discussion
The α-(1 → 3)-glucooligosaccharides obtained via the acid hydrolysis of F. betulina α-(1 → 3)-glucan were characterized by MALDI-TOF MS spectrometry (Fig. 1). The analysis indicated that the hydrolysate contained oligosaccharides composed mainly of 3–6 glucose units associated with a sodium ion ([Glcn + Na]+). Small amounts of disaccharides and oligomers containing from 7 to 15 glucose units were also noticed. In addition, α-(1 → 3)-GOS showed very good solubility in the cell culture medium (10 mg/mL).
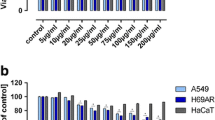
Inhibition of cell division is one of the basic features expected from anticancer agents. In our study, the growth of each cancer cell lines tested was inhibited by α-(1 → 3)-GOS in a dose-dependent manner after 96-h exposure (Fig. 2a–d). The IC50 value of α-(1 → 3)-GOS for the LS180, SW620, SW948, HT-29, and CCD 841 CoTr cell lines was estimated at 19.55, 14.92, 18.30, 17.06, and 39.38 mg/mL, respectively. As can be seen, reduced cancer cell mitochondrial metabolism was observed after 96 h of treatment with α-(1 → 3)-GOS. Interestingly, α-(1 → 3)-GOS exerted an approximately two-fold stronger effect on the cancer than normal colon cells. Additionally, our experiments showed that the early stages (LS180 and HT-29) of colorectal adenocarcinoma were not more sensitive to α-(1 → 3)-GOS than the advanced stages (SW620, SW948).
α-(1 → 3)-GOS influence on the proliferation of colon cells. Cell lines: LS180 (a), SW620 (b), SW948 (c), HT-29 (d), and CCD 841 CoTr (e). The cells were treated with various concentrations of α-(1 → 3)-GOS for 96 h. The cell proliferation of untreated cells (0) was calculated as 100%. Positive control: 10 µM of camptothecin (CPT). The results represent the mean ± SD (n = 5) and were analysed with one-way ANOVA test and Tukey’s Multiple Comparison Post-test (*p < 0.05, **p < 0.01, and ***p < 0.001 were considered statistically significant)
Therefore, the toxic effect of α-(1 → 3)-GOS was evaluated using the human colorectal adenocarcinoma (HT-29), normal human colon epithelial (CCD 841 CoTr), normal mouse skin fibroblast (L929), and normal human skin fibroblasts (HSF) cell lines. The NR assay showed that the viability of all the tested cell lines was unaffected in the entire concentration range (1–10 mg/mL) of α-(1 → 3)-GOS (Fig. 3a–d). The tested α-(1 → 3)-GOS had no cytotoxic activity against the HT-29 cells over the entire concentration range (1–10 mg/ml). However, after the α-(1 → 3)-GOS treatment, the mitochondrial metabolism of the HT-29 cells was significantly reduced in a concentration-dependent manner (Fig. 2d). Thus, it can be assumed that α-(1 → 3)-GOS exhibits antiproliferative activities against colon cancer cells.
α-(1 → 3)-GOS influence on the viability of HT-29 (a), CCD 841 CoTr (b), L929 (c), and HSF (d) cell lines assessed with the NR assay and the CCD 841 CoTr cell line assessed with the LDH assay (e). Positive control: 10 µM of camptothecin (CPT(10 µM)). α-(1 → 3)-GOS at concentrations ranging from 1–10 mg/mL (α-1-3-GOS). α-(1 → 3)-GOS at concentrations ranging from 1–10 mg/mL with 10 µM of camptothecin) (α-1-3-GOS + CPT(10 µM)). The cells were treated for 24 h. The cell viability of untreated cells (0) was calculated as 100%. The results represent the mean ± SD (n = 5) and were analysed with one-way ANOVA test and Tukey’s Multiple Comparison Post-test (*p < 0.05 was considered statistically significant)
The LDH assay data confirmed the lack of α-(1 → 3)-GOS cytotoxicity in the case of the CCD 841 CoTr cells (Fig. 3e). The LDH level was not significantly changed after the exposure to α-(1 → 3)-GOS. However, the treatment of the CCD 841 CoTr cells with α-(1 → 3)-GOS at concentrations of 10 mg/mL with 10 µM of camptothecin caused statistically significant increases in LDH leakage (10 mg/mL of α-(1 → 3)-GOS vs. CPT). We can speculate that 10 mg/mL of the α-(1 → 3)-GOS increases the sensitivity of cells to camptothecin, which consequently disturbs membrane permeability in the CCD 841 CoTr cells more easily. Thus, the α-(1 → 3)-GOS application may lead to increased cytotoxic activity of camptothecin. Additionally, the combination of camptothecin (10 µM) with α-(1 → 3)-GOS (1–7.5 mg/mL) did not significantly disrupt the cell membrane stability, compared to camptothecin alone. Thus, α-(1 → 3)-GOS does not exert a protective effect on the cell membrane of normal CCD 841 CoTr colon cells.
The induction of apoptosis is a good approach for identification of the anticancer potential of the tested compounds. In our study, the 24-h incubation of the HT-29 cancer cells with 10 mg/ml of α-(1 → 3)-GOS induced early and late apoptosis (Table 1). Compared to the untreated cells, the apoptosis rate (combined early and late apoptosis) in the HT-29 cells increased from 15.73% in the control to 21.85% after the 24 h incubation with 10 mg/ml of α-(1 → 3)-GOS. In turn, the apoptosis rate in the CCD 841 CoTr cells remained at a similar level in the same conditions (16.05% and 17.36%, respectively). Since the pro-apoptotic effect was exerted only on HT-29 but not on CCD 841 CoTr after the α-(1 → 3)-GOS treatment, we can presume that there are different mechanisms of its action in cancer and normal colon cells.
Our results reveal the antiproliferative and pro-apoptotic properties of α-(1 → 3)-GOS in a colon cell model. In fact, the pro-apoptotic activity of oligosaccharides and low-molecular-weight α-glucans has been described in the literature. Huang et al. [4] showed that oligosaccharides (stachyose) induced apoptosis and inhibited the growth of Caco-2 colorectal adenocarcinoma cells. In addition, an oligosaccharide isolated and purified from Bombyx batryticatus was found to induce apoptosis and cell cycle arrest in G0/G1 and G2/M phases in HeLa cells [8]. Lavi et al. [10] showed that low-molecular-weight α-glucan isolated from Pleurotus ostreatus inhibited colon cancer cell proliferation and induced apoptosis via an increased level of cytochrome c and Bax. Additionally, Huang et al. [6] showed that sulphated derivatives of α-(1 → 3)-D-glucan induced apoptosis in S-180 (sarcoma) tumour cells by lowering the Bcl-2:Bax ratio. Wiater et al. [19] indicated that a carboxymethylated derivative of α-(1 → 3)-D-glucan decreased the metabolism of cervical carcinoma (HeLa) cells. Sulphated derivatives of α-(1 → 3)-D-glucan have also been found to exhibit antiproliferative activities towards human hepatoma (HepG2) and sarcoma (S-180) cells in vitro [6]. This same authors revealed that a water-soluble phosphated derivative of α-(1 → 3)-D-glucans from Poria cocos mycelia had potent in vivo and in vitro anti-tumour activities [5]. Oligosaccharides have also been shown to have an anticancer effect in the colon environment by stimulation of the immune system [15]. For example, Qamar et al. [14] showed that galacto-oligosaccharides exerted protective effects against pathological pro-cancerous changes in the rat colon by increasing the populations of beneficial bacteria (Bifidobacterium and Lactobacillus) and decreasing the concentrations of harmful strains. In addition, Parish et al. [12] showed that sulphated oligosaccharides with five or more monosaccharides in length were inhibitors of in vitro human angiogenesis and exhibited the antimetastatic activity for rat mammary adenocarcinoma cells.
In conclusion, novel α-(1 → 3)-GOS prepared from F. betulina α-(1 → 3)-glucan showed promising anti-proliferative and pro-apoptotic activity against colon cancer cells. Moreover, α-(1 → 3)-GOS was significantly less effective against normal human colon epithelial cells. Considering our results and the literature data, we support the opinion that α-(1 → 3)-GOS should be investigated in further studies to assess its pro-apoptotic and antiproliferative activity in colorectal cancer models.
References
Choma A, Komaniecka I (2011) Characterization of cyclic β-glucans of Bradyrhizobium by MALDI-TOF mass spectrometry. Carbohydr Res 346:1945–1950. https://doi.org/10.1016/j.carres.2011.05.015
Grienke U, Zöll M, Peintner U, Rollinger JM (2014) European medicinal polypores—A modern view on traditional uses. J Ethnopharmacol 154:564–583. https://doi.org/10.1016/j.jep.2014.04.030
Grün C (2003) Structure and biosynthesis of fungal α -glucans. Dissertation, University of Utrecht. pp 1–144
Huang G, Mao J, Ji Z, Ailati A (2015) Stachyose-induced apoptosis of Caco-2 cells via the caspase-dependent mitochondrial pathway. Food Funct 6:765–771. https://doi.org/10.1039/c4fo01017e
Huang Q, Zhang L (2011) Preparation, chain conformation and anti-tumor activities of water-soluble phosphated (1 → 3)-α-D-glucan from Poria cocos mycelia. Carbohydr Polym 83:1363–1369. https://doi.org/10.1016/J.CARBPOL.2010.09.057
Huang Q, Zhang L, Cheung PCK, Tan X (2006) Evaluation of sulfated α-glucans from Poria cocos mycelia as potential antitumor agent. Carbohydr Polym 64:337–344. https://doi.org/10.1016/J.CARBPOL.2005.12.001
Jelsma J, Kreger DR (1979) Polymorphism in crystalline (1 → 3)-α-d-glucan from fungal cell-walls. Carbohydr Res 71:51–64. https://doi.org/10.1016/S0008-6215(00)86060-7
Jiang X, Zhang Z, Chen Y et al (2014) Structural elucidation and in vitro antitumor activity of a novel oligosaccharide from Bombyx batryticatus. Carbohydr Polym 103:434–441. https://doi.org/10.1016/j.carbpol.2013.12.039
Kim S-K, Rajapakse N (2005) Enzymatic production and biological activities of chitosan oligosaccharides (COS): a review. Carbohydr Polym 62:357–368. https://doi.org/10.1016/J.CARBPOL.2005.08.012
Lavi I, Friesem D, Geresh S et al (2006) An aqueous polysaccharide extract from the edible mushroom Pleurotus ostreatus induces anti-proliferative and pro-apoptotic effects on HT-29 colon cancer cells. Cancer Lett 244:61–70. https://doi.org/10.1016/J.CANLET.2005.12.007
Lindequist U, Niedermeyer THJ, Jülich W-D (2005) The pharmacological potential of mushrooms. Evid Based Complement Altern Med 2:285–299. https://doi.org/10.1093/ecam/neh107
Parish CR, Freeman C, Brown KJ et al (1999) Identification of sulfated oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase activity. Cancer Res 59:3433–3441
Pleszczyńska M, Lemieszek MK, Siwulski M et al (2017) Fomitopsis betulina (formerly Piptoporus betulinus): the Iceman’s polypore fungus with modern biotechnological potential. World J Microbiol Biotechnol 33:83. https://doi.org/10.1007/s11274-017-2247-0
Qamar TR, Iqbal S, Syed F et al (2017) Impact of novel prebiotic galacto-oligosaccharides on various biomarkers of colorectal cancer in wister rats. Int J Mol Sci. https://doi.org/10.3390/ijms18091785
Raman M, Ambalam P, Kondepudi KK et al (2013) Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes 4:181–192. https://doi.org/10.4161/gmic.23919
Rumney C, Rowland I (1995) Non-digestible oligosaccharides—potential anticancer agents? Nutr Bull 20:194–203. https://doi.org/10.1111/j.1467-3010.1995.tb00605.x
Stepanenko AA, Dmitrenko VV (2015) Pitfalls of the MTT assay: direct and off-target effects of inhibitors can result in over/underestimation of cell viability. Gene 574:193–203. https://doi.org/10.1016/j.gene.2015.08.009
Tsao CT, Chang CH, Lin YY et al (2011) Kinetic study of acid depolymerization of chitosan and effects of low molecular weight chitosan on erythrocyte rouleaux formation. Carbohydr Res 346:94–102. https://doi.org/10.1016/J.CARRES.2010.10.010
Wiater A, Paduch R, Choma A et al (2012) Biological study on carboxymethylated (1 → 3)-α-d-glucans from fruiting bodies of Ganoderma lucidum. Int J Biol Macromol 51:1014–1023. https://doi.org/10.1016/J.IJBIOMAC.2012.08.017
Wiater A, Paduch R, Pleszczyńska M et al (2011) α-(1 → 3)-D-glucans from fruiting bodies of selected macromycetes fungi and the biological activity of their carboxymethylated products. Biotechnol Lett 33:787–795. https://doi.org/10.1007/s10529-010-0502-7
Zamłyńska K, Komaniecka I, Żebracki K et al (2017) Studies on lipid A isolated from Phyllobacterium trifolii PETP02T lipopolysaccharide. Antonie Van Leeuwenhoek 110:1413–1433. https://doi.org/10.1007/s10482-017-0872-0
Zhu F, Du B, Bian Z, Xu B (2015) Beta-glucans from edible and medicinal mushrooms: characteristics, physicochemical and biological activities. J Food Compos Anal 41:165–173. https://doi.org/10.1016/J.JFCA.2015.01.019
Acknowledgements
This work was supported by statutory funding of Maria Curie-Skłodowska University. The Waters Synapt G2-Si HDMS spectrometer was purchased from the European Union Funds, Operating Programme Infrastructure and Environment (project number: UDA-POiS.13.01-045/08).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Czerwonka, A., Wiater, A., Komaniecka, I. et al. Antitumour effect of glucooligosaccharides obtained via hydrolysis of α-(1 → 3)-glucan from Fomitopsis betulina. Mol Biol Rep 46, 5977–5982 (2019). https://doi.org/10.1007/s11033-019-05032-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-05032-x