Abstract
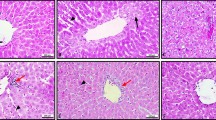
Excessive alcohol consumption and alcoholism cause medical problems with high mortality and morbidity rates. In this study we aimed to decrease the alcohol related tissue damage by inhibiting calpain activation which plays an important role in apoptosis and necrosis, in rats with cardiomyopathy induced by acute alcohol consumption. Male Sprague–Dawley rats were separated into four groups (control, vehicle, alcohol and alcohol + inhibitor) with 10 rats in each. Control group received isocaloric maltose while vehicle group received isocaloric maltose with DMSO, and alcohol group received 8 g/kg absolute ethanol by gavage. Inhibitor group received 20 mg/kg calpain inhibitor 1 intraperitonally prior to alcohol administration. Calpain activities, cathepsin L levels and cytochrome c release rates were significantly increased in alcohol group compared to control group (p < 0.05). Serum CK MB and BNP levels of alcohol group were excessively increased compared to control group (respectively p < 0.001 and p < 0.01). Serum BNP levels of alcohol + inhibitor group were significantly (p < 0.05) decreased compared to alcohol group. In addition to these, histological evaluation of light microscope images and the results of DNA fragmentation and immunohistochemical caspase-3 activity results showed significant improvement of these parameters in alcohol + inhibitor group compared to alcohol group. Results of our biochemical and histological evaluation results revealed that the calpain inhibitor N-acetyl-leu-leu-norleucinal may have an ameliorating effect on acute alcohol consumption related cardiac tissue damage due to its effects on cell death pathways.





Similar content being viewed by others
References
Fernandez-Sola J, Estruch R, Grau JM, Pare JC, Rubin E, Urbano-Marquez A (1994) The relation of alcoholic myopathy to cardiomyopathy. Ann Intern Med 120:529–536
Zhang D, Gaussin V, Taffet GE et al (2000) TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med 6:556–563
Guo R, Ren J (2010) Alcohol dehydrogenase accentuates ethanol-induced myocardial dysfunction and mitochondrial damage in mice: role of mitochondrial death pathway. PLoS One 18:8757
Kar P, Samanta K, Shaikh S, Chowdhury A, Chakraborti T, Chakraborti S (2010) Mitochondrial calpain system: an overview. Arch Biochem Biophys 495:1–7
Kuralay F, Çavdar Z (2006) İnflamatuar medyatörlere toplu bir bakış. Genel Tıp Derg 16:143–152
Rajgopal Y, Vemuri MC (2002) Calpain activation and a-spectrin cleavage in rat brain by ethanol. Neurosci Lett 321:187–191
Blomgren K, Zhu C, Wang X et al (2001) Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathological apoptosis”? J Biol Chem 276:10191–10198
Syntichaki P, Tavernarakis N (2003) The biochemistry of neuronal necrosis: rogue biology. Nat Rev Neurosci 4:672–684
Donohue TM, Curry-McCoy TV, Nanji AA et al (2007) Lysosomal leakage and lack of adaptation of hepatoprotective enzyme contribute to enhanced susceptibility to ethanol-induced liver injury in female rats. Alcohol Clin Exp Res 31:1944–1952
Repnik U, Turk B (2010) Lysosomal–mitochondrial cross-talk during cell death. Mitochondrion 10:662–669
Ge J, Zhao G, Chen R et al (2006) Enhanced myocardial cathepsin B expression in patients with dilated cardiomyopathy. Eur J Heart Fail 8:284–289
Tsuchida K, Aihara H, Isogai K, Hanada K, Shibata N (1986) Degradation of myocardial structural proteins in myocardial infarcted dogs is reduced by Ep459, a cysteine proteinase inhibitor. Biol Chem Hoppe Seyler 367:39–45
Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol Rev 83:731–801
Cuzzocrea S, McDonald MC, Mazzon E et al (2000) Calpain inhibitor I reduces the development of acute and chronic inflammation. Am J Pathol 157:2065–2079
Yoshikawa Y, Hagihara H, Ohga Y et al (2005) Calpain inhibitor-1 protects the rat heart from ischemia–reperfusion injury: analysis by mechanical work and energetic. Am J Physiol Heart Circ Physiol 288:1690–1698
Chatterjee PK, Brown PA, Cuzzocrea S et al (2001) Calpain inhibitor-1 reduces renal ischemia/reperfusion injury in the rat. Kidney Int 59:2073–2083
Sandmann S, Prenzel F, Shaw L, Schauer R, Unger T (2002) Activity profile of calpains I and II in chronically infarcted rat myocardium–influence of the calpain inhibitor CAL 9961. Br J Pharmacol 135:1951–1958
Mani SK, Shiraishi H, Balasubramanian S et al (2008) In vivo administration of calpeptin attenuates calpain activation and cardiomyocyte loss in pressure-overloaded feline myocardium. Am J Physiol Heart Circ Physiol 295:314–326
Chandrasekhar R, Huang HM, Sun GY (1988) Alterations in rat brain polyphosphoinositide metabolism due to acute ethanol administration. J Pharmacol Exp Ther 245:120–123
Kannan M, Wang L, Kang YJ (2004) Myocardial oxidative stress and toxicity induced by acute ethanol exposure in mice. Exp Biol Med 229:553–559
Sheeba MS, Asha VV (2006) Effect of Cardiospermum halicacabum on ethanol-induced gastric ulcers in rats. J Ethnopharmacol 106:105–110
Jump SS, Childs TE, Zwetsloot KA, Booth FW, Lees SJ (2009) Fibroblast growth factor 2-stimulated proliferation is lower in muscle precursor cells from old rats. Exp Physiol 94:739–748
Li SY, Fang CX, Aberle NS 2nd, Ren BH, Ceylan-Isik AF, Ren J (2005) Inhibition of PI-3 kinase/Akt/mTOR, but not calcineurin signaling, reverses insulin-like growth factor I-induced protection against glucose toxicity in cardiomyocyte contractile function. J Endocrinol 186:491–503
McDonald MC, Mota-Filipe H, Paul A et al (2001) Calpain inhibitor I reduces the activation of nuclear factor-kB and organ injury/dysfunction in hemorrhagic shock. FASEB J 15:171–186
Işlekel H, Işlekel S, Güner G, Ozdamar N (1999) Evaluation of lipid peroxidation, cathepsin L and acid phosphatase activities in experimental brain ischemia–reperfusion. Brain Res 843:18–24
Barrett AJ, Kirschke H (1981) Methods Enzymol 80:535–538
Zovein A, Flowers-Ziegler J, Thamotharan S et al (2004) Postnatal hypoxic-ischemic brain injury alters mechanisms mediating neuronal glucose transport. Am J Physiol Regul Integr Comp Physiol 286:273–282
Soeda J, Miyagawa S, Sano K, Masumoto J, Taniguchi S, Kawasaki S (2001) Cytochrome c release into cytosol with subsequent caspase activation during warm ischemia in rat live. Am J Physiol Gastrointest Liver Physiol 281:1115–1123
Grunnet LG, Aikin R, Tonnesen MF et al (2009) Proinflammatory cytokines activate the intrinsic apoptotic pathway in β-cells. Diabetes 58:1807–1815
Bradford MM (1976) A rapid and sensitive for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burns MJ, Dickson EW, Sivilotti ML, Cuenoud H (2001) Phentolamine reduces myocardial injury and mortality in a rat model of phenylpropanolamine poisoning. J Toxicol Clin Toxicol 39:129–134
Fenwick MA, Hurst PR (2002) Immunohistochemical localization of active caspase-3 in the mouse ovary: growth and atresia of small follicles. Reproduction 124:659–665
Piano MR (2002) Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest 121:1638–1650
Krenz M, Cohen MV, Downey JM (2002) The protective and anti-protective effects of ethanol in a myocardial infarct model. Ann NY Acad Sci 957:103–114
Capasso JM, Li P, Guideri G (1992) Myocardial mechanical, biochemical and structural alterations induced by chronic ethanol ingestion in rats. Circ Res 71:346–356
Chen DB, Wang L, Wang PH (2000) Insulin-like growth factor I retards apoptotic signaling induced by ethanol in cardiomyocytes. Life Sci 67:1683–1693
Berridge MJ, Bootman MD, Lipp P (1998) Calcium, a life and death signal. Nature 395:645–648
Clapham DE (1995) Calcium signalling. Cell 80:259–268
Arthur GD, Belcastro AN (1997) A calcium stimulated cysteine protease is involved in isoproterenol induced cardiac hypertrophy. Mol Cell Biochem 176:241–248
Delbridge LM, Connell PJ, Harris PJ, Morgan TO (2000) Ethanol effects on cardiomyocyte contractility. Clin Sci 98:401–407
Kawada T, Masui F, Kumagai H, Koshimizu M, Nakazawa M, Toyo-Oka T (2005) A novel paradigm for therapeutic basis of advanced heart failure–assessment by gene therapy. Pharmacol Ther 107:31–43
Khalil PN, Neuhof C, Huss R et al (2005) Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. Eur J Pharmacol 528:124–131
Okada K, Minamino T, Tsukamoto Y et al (2004) Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation 110:705–712
Saitoh T, Nakajima T, Takahashi T, Kawahara K (2006) Changes in cardiovascular function on treatment of inhibitors of apoptotic signal transduction pathways in left ventricular remodeling after myocardial infarction. Cardiovasc Pathol 15:130–138
Singh RB, Dandekar SP, Elimban V, Gupta SK, Dhalla NS (2004) Role of proteases in the pathophysiology of cardiac disease. Mol Cell Biochem 263:241–256
Takahashi M, Tanonaka K, Yoshida H et al (2006) Possible involvement of calpain activation in pathogenesis of chronic heart failure after acute myocardial infarction. J Cardiovasc Pharmacol 47:413–421
Sun AY, Ingelman-Sundberg M, Neve E et al (2001) Ethanol and oxidative stress. Alcohol Clin Exp Res 25:237–243
Zhao M, Antunes F, Eaton JW, Brunk UT (2003) Lysosomal enzymes promote mitochondrial oxidant production, cytochrome c release and apoptosis. Eur J Biochem 270:3778–3786
Reilly ME, Mantle D, Salisbury J, Peters TJ, Preedy VR (2000) Comparative effects of acute ethanol dosage on liver and muscle protein metabolism. Biochem Pharmacol 60:1773–1785
Gill C, Mestril R, Samali A (2002) Losing heart: the role of apoptosis in heart disease a novel therapeutic target. FASEB J 16:135–146
Burgess DH, Svensson M, Dandrea T et al (1999) Human skeletal muscle cytosols are refractory to cytochrome c-dependent activation of type-II caspases and lack APAF-1. Cell Death Differ 6:256–261
Kelkar S, Dong Q, Xiao Y, Joshi-Barve S, McClain CJ, Barve SS (2002) Ethanol enhances activation-induced caspase-3 dependent cell death in T lymphocytes. Alcohol Clin Exp Res 26:363–370
Olney JW, Tenkova T, Dikranian K, Muglia LJ, Jermakowicz WJ, D’Sa C (2002) Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis 9:205–219
Young C, Roth KA, Klocke BJ et al (2005) Role of caspase-3 in ethanol-induced developmental neurodegeneration. Neurobiol Dis 20:608–614
Ray SK, Matzella DD, Wilford EL (2001) Inhibition of calpain mediated apoptosis by E-64-d reduced immediate early gene expression and reaktive astrogliosis in the lesion and penumbra following spinal cord injury in rats. Brain Res 916:115–126
Swapan KR, Edward LH, Naren LB (2003) Calpain in the pathophysiology of spinal cord injury: neuroprotection with calpain inhibitors. Brain Res Rev 42:169–185
Acknowledgments
This study was funded by Research Projects, Commission of Osmangazi University (Project No. 201011003).
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kartkaya, K., Kanbak, G., Oğlakçı, A. et al. Protective effect of calpain inhibitor N-acetyl-l-leucyl-l-leucyl-l-norleucinal on acute alcohol consumption related cardiomyopathy. Mol Biol Rep 41, 6743–6753 (2014). https://doi.org/10.1007/s11033-014-3560-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3560-4