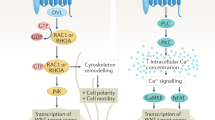
Abstract
Wnts are secreted glycoproteins implicated in biological processes ranging from embryonic cardiac development to uncontrolled cell proliferation in diseased conditions. Cardiovascular disease is a major cause of morbidity and mortality worldwide. Phenotypic modulation of vascular smooth muscle cells, migration and proliferation in intimal layer and increased extracellular matrix production are some of the known hallmarks of cardiovascular pathologies. Heterogeneity associated with the binding of Wnts to their transmembrane receptors, Frizzled, and coreceptors low density lipoprotein-receptor-related protein is indeed intriguing. Nuclear-cytoplasmic shuttling of beta-catenin and activation of transcriptional factors, lymphoid enhancer factor and T cell activation factor leading to target gene activation has remained elusive. Our review highlights the emerging role of Wnt-Frizzled signaling in cardiovascular diseases. Overall, the pathway appears to be an attractive therapeutic target in identifying susceptible individuals at risk of developing restenosis/other vascular pathologies in the near future.

Similar content being viewed by others
Abbreviations
- APC:
-
Adenomatous polyposis coli
- β-cat:
-
Beta-catenin
- CK:
-
Casein kinase
- [Ca]i:
-
Intracellular calcium
- CamK:
-
Calmodulin dependent protein kinase
- DAG:
-
Diacyl glycerol
- Dsh:
-
Dishevelled
- Fzd:
-
Frizzled
- GSK:
-
Glycogen synthase kinase
- IP3 :
-
Inositol-tri-phosphate
- LEF:
-
Lymphoid enhancer factor
- LRP:
-
Low density-lipoprotein-receptor related protein
- PKC:
-
Protein kinase C
- PLC:
-
Phospholipase C
- TCF:
-
T cell activation factor
- Wnt:
-
Drosophila Wingless and mouse Int-1
References
Brade T, Männer J, Kühl M (2006) The role of Wnt signalling in cardiac development and tissue remodeling in the mature heart. Cardiovasc Res 72:198–209
Nusse R, Varmus HE (1982) Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 31:99–109
Nusse R, Varmus H (2012) Three decades of Wnts: a personal perspective on how a scientific field developed. EMBO J 31:2670–2684
Li F, Chong ZZ, Maiese K (2006) Winding through the WNT pathway during cellular development and demise. Histol Histopathol 21:103–124
Miller JR (2002) The Wnts. Genome Biol 3:S3001
Bejsovec A (2006) Flying at the head of the pack: Wnt biology in Drosophila. Oncogene 25:7442–7449
Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R (1996) A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature 382:225–230
Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S (2000) Arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature 407:527–530
Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127:469–480
Kohn AD, Moon RT (2005) Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium 38:439–446
Katoh M (2005) WNT/PCP signaling pathway and human cancer (review). Oncol Rep 14:1583–1588
Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR 3rd, Nusse R (2003) Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423:448–452
Zhai L, Chaturvedi D, Cumberledge S (2004) Drosophila wnt-1 undergoes a hydrophobic modification and is targeted to lipid rafts, a process that requires porcupine. J Biol Chem 279:33220–33227
Pandey S, Murphy RF, Agrawal DK (2007) Recent advances in the immunobiology of ceramide. Exp Mol Pathol 82:298–309
Coudreuse DY, Roël G, Betist MC, Destrée O, Korswagen HC (2006) Wnt gradient formation requires retromer function in Wnt-producing cells. Science 312:921–924
Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC (2000) An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407:535–538
Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X (2000) LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530–535
Mao J, Wang J, Liu B, Pan W, Farr GH 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D (2001) Low density lipoprotein-receptor-related protein-5 binds to axin and regulates the canonical Wnt signaling pathway. Mol Cell 7:801–809
George SJ (2008) Wnt pathway: a new role in regulation of inflammation. Arterioscler Thromb Vasc Biol 28:400–402
Polakis P (2000) Wnt signaling and cancer. Genes Dev 14:1837–1851
Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP (2007) LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science 315:1278–1282
Mi K, Johnson GV (2007) Regulated proteolytic processing of LRP6 results in release of its intracellular domain. J Neurochem 101:517–529
Hsieh JC (2000) Specificity of WNT–receptor interactions. Front Biosci 9:1333–1338
Wang Y, Macke JP, Abella BS, Andreasson K, Worley P, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J (1996) A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J Biol Chem 271:4468–4476
Adler PN, Vinson C, Park WJ, Conover S, Klein L (1990) Molecular structure of frizzled, a Drosophila tissue polarity gene. Genetics 126:401–416
Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A (1999) DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol 19:4414–4422
Caira FC, Stock SR, Gleason TG, Mc Gee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM (2006) Human degenerative valve disease is associated with up 12 regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol 47:1707–1712
Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J (2003) Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell 12:1251–1260
Sakanaka C, Leong P, Xu L, Harrison SD, Williams LT (1999) Casein kinase I-epsilon in the wnt pathway: regulation of beta-catenin function. Proc Natl Acad Sci USA 96:12548–12552
Peters JM, McKay RM, McKay JP, Graff JM (1999) Casein kinase I transduces Wnt signals. Nature 401:345–350
Peifer M, Polakis P (2000) Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287:1606–1609
Behrens J, Jerchow BA, Würtele M, Grimm J, Asbrand C, Wirtz R, Kühl M, Wedlich D, Birchmeier W (1998) Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280:596–599
Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P (1998) Downregulation of beta-catenin by human axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol 8:573–581
Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A (1998) Axil, a member of the axin family, interacts with both glycogen synthase kinase 3beta and beta-catenin and inhibits axis formation of Xenopus embryos. Mol Cell Biol 18:2867–2875
Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A (1998) Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J 17:1371–1384
Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F (1997) The mouse Fused locus encodes axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90:181–192
Henderson BR (2000) Nuclear-cytoplasmic shuttling of APC regulates beta-catenin subcellular localization and turnover. Nat Cell Biol 2:653–660
Henderson BR, Fagotto F (2002) The ins and outs of APC and beta-catenin nuclear transport. EMBO Rep 3:834–839
Price MA (2006) CKI, there’s more than one: casein kinase I family members in Wnt and Hedgehog signaling. Genes Dev 20:399–410
Zhang N, Jiang Y, Zou J, Zhuang S, Jin H, Yu Q (2007) Insights into unbinding mechanisms upon two mutations investigated by molecular dynamics study of GSK3beta–axin complex: role of packing hydrophobic residues. Proteins 67:941–949
Kühl M, Sheldahl LC, Park M, Miller JR, Moon RT (2000) The Wnt/Ca2+ pathway: a new vertebrate Wnt signaling pathway takes shape. Trends Genet 16:279–283
Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8:645–654
Arce L, Yokoyama NN, Waterman ML (2006) Diversity of LEF/TCF action in development and disease. Oncogene 25:7492–7504
van Noort M, Clevers H (2000) TCF transcription factors, mediators of Wnt-signaling in development and cancer. Dev Biol 244:1–8
Lustig B, Behrens J (2003) The Wnt signaling pathway and its role in tumor development. J Cancer Res Clin Oncol 129:199–221
Städeli R, Hoffmans R, Basler K (2006) Transcription under the control of nuclear Arm/beta catenin. Curr Biol 16:R378–R385
Cohen AW, Carbajal JM, Schaeffer RC Jr (1999) VEGF stimulates tyrosine phosphorylation of beta-catenin and small-pore endothelial barrier dysfunction. Am J Physiol 277:H2038–H2049
Dono R, Faulhaber J, Galli A, Zuniga A, Volk T, Texido G, Zeller R, Ehmke H (2002) FGF2 signaling is required for the development of neuronal circuits regulating blood pressure. Circ Res 90:E5–E10
Libby P, Simon DI (2001) Inflammation and thrombosis: the clot thickens. Circulation 103:1718–1720
Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA (1996) Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood 88:146–157
Holnthoner W, Pillinger M, Groger M, Wolff K, Ashton AW, Albanese C, Neumeister P, Pestell RG, Petzelbauer P (2002) Fibroblast growth factor-2 induces Lef/Tcf-dependent transcription in human endothelial cells. J Biol Chem 277:45847–45853
Mitra AK, Agrawal DK (2006) In stent restenosis: bane of the stent era. J Clin Pathol 59:232–239
Rajamannan NM (2011) The role of Lrp5/6 in cardiac valve disease: LDL-density–pressure theory. J Cell Biochem 112:2222–2229
Lyon C, Mill C, Tsaousi A, Williams H, George S (2011) Regulation of VSMC behavior by the cadherin–catenin complex. Front Biosci 16:644–657
Naito AT, Shiojima I, Komuro I (2010) Wnt signaling and aging-related heart disorders. Circ Res 107(1295):1303
Yeo EL, Sheppard JA, Feuerstein IA (1994) Role of P-selectin and leukocyte activation in polymorphonuclear cell adhesion to surface adherent activated platelets under physiologic shear conditions (an injury vessel wall model). Blood 83:2498–2507
Inoue T, Sakai Y, Hoshi K, Yaguchi I, Fujito T, Morooka S (1998) Lower expression of neutrophil adhesion molecule indicates less vessel wall injury and might explain lower restenosis rate after cutting balloon angioplasty. Circulation 97:2511–2518
Furukawa Y, Matsumori A, Ohashi N, Shioi T, Ono K, Harada A, Matsushima K, Sasayama S (1999) Anti monocyte chemoattractant protein-1/monocyte chemotactic and activating factor antibody inhibits neointimal hyperplasia in injured rat carotid arteries. Circ Res 84:306–314
Cipollone F, Marini M, Fazia M, Pini B, Iezzi A, Reale M, Paloscia L, Materazzo G, D’Annunzio E, Conti P, Chiarelli F, Cuccurullo F, Mezzetti A (2001) Elevated circulating levels of monocyte chemoattractant protein-1 in patients with restenosis after coronary angioplasty. Arterioscler Thromb Vasc Biol 21:327–334
Belema Bedada F, Technau A, Ebelt H, Schulze M, Braun T (2005) Activation of myogenic differentiation pathways in adult bone marrow-derived stem cells. Mol Cell Biol 25:9509–9519
Haq S, Michael A, Andreucci M, Bhattacharya K, Dotto P, Walters B, Woodgett J, Kilter H, Force T (2003) Stabilization of beta-catenin by a Wnt-independent mechanism regulates cardiomyocyte growth. Proc Natl Acad Sci USA 100:4610–4615
Blankesteijn WM, Essers-Janssen YP, Verluyten MJ, Daemen MJ, Smits JF (1997) A homologue of Drosophila tissue polarity gene frizzled is expressed in migrating myofibroblasts in the infarcted rat heart. Nat Med 3:541–544
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C (1998) Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391:357–362
Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, Glinka A, Niehrs C (2002) Kremen proteins are Dickkopf receptors that regulate Wnt/beta-catenin signalling. Nature 417:664–667
Takemaru K, Yamaguchi S, Lee YS, Zhang Y, Carthew RW, Moon RT (2003) Chibby, a nuclear beta-catenin-associated antagonist of the Wnt/Wingless pathway. Nature 422:905–909
Schachner T (2006) Pharmacologic inhibition of vein graft neointimal hyperplasia. J Thorac Cardiovasc Surg 131:1065–1072
Taylor AM, Li F, Thimmalapura P, Gerrity RG, Sarembock IJ, Forrest S, Rutherford S, McNamara CA (2006) Hyperlipemia and oxidation of LDL induce vascular smooth muscle cell growth: an effect mediated by the HLH factor Id3. J Vasc Res 43:123–130
Krueger KD, Mitra AK, DelCore MG, Hunter WJ 3rd, Agrawal DK (2006) A comparison of stent induced stenosis in coronary and peripheral arteries. J Clin Pathol 59:575–579
Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V (2007) Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA 104:1643–1648
George A, Leahy H, Zhou J, Morin PJ (2007) The vacuolar-ATPase inhibitor bafilomycin and mutant VPS35 inhibit canonical Wnt signaling. Neurobiol Dis 26:125–133
Flaherty MP, Dawn B (2008) Noncanonical Wnt11 signaling and cardiomyogenic differentiation. Trends Cardiovasc Med 18:260–268
Mill C, George SJ (2012) Wnt signalling in smooth muscle cells and its role in cardiovascular disorders. Cardiovasc Res 95:233
Lyon C, Mill C, Tsaousi A, Williams H, George S (2011) Regulation of VSMC behavior by the cadherin-catenin complex. Front Biosci 16:644–657
Tsaousi A, Williams H, Lyon CA, Taylor V, Swain A, Johnson JL, George SJ (2011) Wnt4/β-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ Res 108:427–436
Tsaousi A, Mill C, George SJ (2011) The Wnt pathways in vascular disease: lessons from vascular development. Curr Opin Lipidol 22:350–357
Rao TP, Kühl M (2010) An updated overview on Wnt signaling pathways: a prelude for more. Circ Res 106:1798–1806
van de Schans VA, Smits JF, Blankesteijn WM (2008) The Wnt/frizzled pathway in cardiovascular development and disease: friend or foe? Eur J Pharmacol 585:338–345
Blankesteijn WM, van de Schans VA, ter Horst P, Smits JF (2008) The Wnt/frizzled/GSK-3 beta pathway: a novel therapeutic target for cardiac hypertrophy. Trends Pharmacol Sci 29:175–180
Acknowledgments
We have not received any payment/honoraria in the preparation of this manuscript from Government funding agencies or Corporate/Industrial sponsor. We apologise to colleagues in the Wnt/vascular field(s) whose work may not have been cited due to space reasons.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, S., Chandravati Targeting Wnt-Frizzled signaling in cardiovascular diseases. Mol Biol Rep 40, 6011–6018 (2013). https://doi.org/10.1007/s11033-013-2710-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-2710-4