Abstract
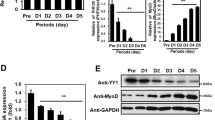
Six1 protein belongs to the Six homeoproteins family, exposing typical domain structure. Although the functions of Six1 have been drawn much attention, the roles of its individual domains are not completely elucidated. Here, we first detected the expression patterns of myogenin, MyoD, Myf5, and Six1 genes using real-time PCR in differentiating C2C12 cells cultured in differentiation medium for 2 or 6 days. The results showed that Six1 gene had the similar expression pattern with myogenin, MyoD, and Myf5 genes, which suggests that it may affect the myogenic differentiation. In order to evaluate the role of distinct domains of Six1 protein in subcellular localization, we constructed a series of truncated vectors tagged with green fluorescent proteins expressing various regions of porcine Six1 protein for subcellular localization analysis. Fluorescence confocal microscopy analysis showed that the different regions of Six1 protein displayed discrete distributions throughout the nucleus and the cytoplasm. The full-length CDS was exclusively localized in the nucleus and the individual HD domain was preferentially distributed to the nucleus both in C2C12 cells and in PK cells. However, the SD domain was diffusely distributed to the cytoplasm and the nucleus, and the localization of SD domain was biased to cytoplasm in C2C12 cells. Taken together, we conclude that the HD domain is important for the nuclear localization of porcine Six1 protein.



Similar content being viewed by others
References
Seo HC, Curtiss J, Mlodzik M, Fjose A (1999) Six class homeobox genes in drosophila belong to three distinct families and are involved in head development. Mech Dev 83(1–2):127–139. doi:10.1016/S0925-4773(99)00045-3
Kawakami K, Sato S, Ozaki H, Ikeda K (2000) Six family genes-structure and function as transcription factors and their roles in development. BioEssays 22(7):616–626. doi:10.1002/1521-1878(200007)22:7<616:AID-BIES4>3.0.CO;2-R
Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, Maire P (1998) Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci USA 95(24):14220–14225. doi:10.1073/pnas.95.24.14220
Giordani J, Bajard L, Demignon J, Daubas P, Buckingham M, Maire P (2007) Six proteins regulate the activation of Myf5 expression in embryonic mouse limbs. Proc Natl Acad Sci USA 104(27):11310–11315. doi:10.1073/pnas.0611299104
Grifone R, Laclef C, Spitz F, Lopez S, Demignon J, Guidotti JE, Kawakami K, Xu PX, Kelly R, Petrof BJ, Daegelen D, Concordet JP, Maire P (2004) Six1 and Eya1 expression can reprogram adult muscle from the slow-twitch phenotype into the fast-twitch phenotype. Mol Cell Biol 24(14):6253–6267. doi:10.1128/MCB.24.14.6253-6267.2004
Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG (2003) Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature 426(6964):247–254. doi:10.1038/Nature02083
Laclef C, Hamard G, Demignon J, Souil E, Houbron C, Maire P (2003) Altered myogenesis in Six1-deficient mice. Development 130(10):2239–2252. doi:10.1242/dev.00440
Xu PX, Zheng WM, Huang L, Maire P, Laclef C, Silvius D (2003) Six1 is required for the early organogenesis of mammalian kidney. Development 130(14):3085–3094. doi:10.1242/Dev.00536
Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, Okamura H, Kitamura K, Muto S, Kotaki H, Sudo K, Horai R, Iwakura Y, Kawakami K (2004) Six1 controls patterning of the mouse otic vesicle. Development 131(3):551–562. doi:10.1242/Dev.00943
Grifone R, Demignon J, Houbron C, Souil E, Niro C, Seller MJ, Hamard G, Maire P (2005) Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development 132(9):2235–2249. doi:10.1242/dev.01773
Bessarab DA, Chong SW, Srinivas BP, Korzh V (2008) Six1a is required for the onset of fast muscle differentiation in zebrafish. Dev Biol 323(2):216–228. doi:10.1016/j.ydbio.2008.08.015
Niro C, Demignon J, Vincent S, Liu YB, Giordani J, Sgarioto N, Favier M, Guillet-Deniau I, Blais A, Maire P (2010) Six1 and Six4 gene expression is necessary to activate the fast-type muscle gene program in the mouse primary myotome. Dev Biol 338(2):168–182. doi:10.1016/j.ydbio.2009.11.031
Wu W, Ren Z, Wang Y, Chao Z, Xu D, Xiong Y (2011) Molecular characterization, expression patterns and polymorphism analysis of porcine Six1 gene. Mol Biol Rep 38(4):2619–2632. doi:10.1007/s11033-010-0403-9
Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K (1999) Cooperation of Six and Eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol 19(10):6815–6824
Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL (1997) The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell 91(7):881–891. doi:S0092-8674(00)80480-8
Heanue T, Reshef R, Davis R, Mardon G, Oliver G, Tomarev S, Lassar A, Tabin C (1999) Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev 13:3231–3243
Rudnicki MA, Braun T, Hinuma S, Jaenisch R (1992) Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell 71(3):383–390. doi:0092-8674(92)90508-A
Braun T, Rudnicki MA, Arnold HH, Jaenisch R (1992) Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell 71(3):369–382. doi:0092-8674(92)90507-9
Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R (1993) MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75(7):1351–1359. doi:0092-8674(93)90621-V
Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, Klein WH (1993) Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature 364(6437):501–506. doi:10.1038/364501a0
Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I (1993) Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature 364(6437):532–535. doi:10.1038/364532a0
Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M (1997) Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell 89(1):127–138. doi:S0092-8674(00)80189-0
Kablar B, Krastel K, Ying C, Tapscott SJ, Goldhamer DJ, Rudnicki MA (1999) Myogenic determination occurs independently in somites and limb buds. Dev Biol 206(2):219–231. doi:10.1006/dbio.1998.9126
Weintraub H (1993) The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell 75(7):1241–1244. doi:0092-8674(93)90610-3
Kucharczuk KL, Love CM, Dougherty NM, Goldhamer DJ (1999) Fine-scale transgenic mapping of the MyoD core enhancer: MyoD is regulated by distinct but overlapping mechanisms in myotomal and non-myotomal muscle lineages. Development 126(9):1957–1965
Buchberger A, Nomokonova N, Arnold HH (2003) Myf5 expression in somites and limb buds of mouse embryos is controlled by two distinct distal enhancer activities. Development 130(14):3297–3307. doi:10.1242/dev.00557
Chen JC, Goldhamer DJ (1999) Transcriptional mechanisms regulating MyoD expression in the mouse. Cell Tissue Res 296(1):213–219. doi:10.1007/s004410051282
Bajard L, Relaix F, Lagha M, Rocancourt D, Daubas P, Buckingham ME (2006) A novel genetic hierarchy functions during hypaxial myogenesis: Pax3 directly activates Myf5 in muscle progenitor cells in the limb. Genes Dev 20(17):2450–2464. doi:10.1101/gad.382806
Maroto M, Reshef R, Munsterberg AE, Koester S, Goulding M, Lassar AB (1997) Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell 89(1):139–148. doi:10.1016/S0092-8674(00)80190-7
Bhagavati S, Song X, Siddiqui MA (2007) RNAi inhibition of Pax3/7 expression leads to markedly decreased expression of muscle determination genes. Mol Cell Biochem 302(1–2):257–262. doi:10.1007/s11010-007-9444-3
Fougerousse F, Durand M, Lopez S, Suel L, Demignon J, Thornton C, Ozaki H, Kawakami K, Barbet P, Beckmann JS, Maire P (2002) Six and Eya expression during human somitogenesis and MyoD gene family activation. J Muscle Res Cell Motil 23(3):255–264. doi:10.1023/A:1020990825644
Kawakami K, Ohto H, Ikeda K, Roeder RG (1996) Structure, function and expression of a murine homeobox protein AREC3, a homologue of Drosophila sine oculis gene product, and implication in development. Nucleic Acids Res 24(2):303–310. doi:10.1093/nar/24.2.303
Acknowledgments
This work was supported by National Natural Science Foundation of China (31000996) and the National Project for Breeding of Transgenic Pig (2008ZX08006-002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Wangjun Wu and Zhuqing Ren contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, W., Ren, Z., Chen, C. et al. Subcellular localization of different regions of porcine Six1 gene and its expression analysis in C2C12 myoblasts. Mol Biol Rep 39, 9995–10002 (2012). https://doi.org/10.1007/s11033-012-1868-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-012-1868-5