Abstract
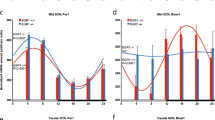
Most living organisms exhibit circadian rhythms in physiology and behavior. These oscillations are generated by an endogenous circadian clock and control many biological processes. Ceramide has attracted attention as a signal mediator in diverse cell processes including cell death and differentiation. The relationships between ceramide expression levels and the circadian clock have not previously been investigated. To determine if there are circadian variations in the content of ceramide, we measured ceramide concentrations in the livers of wild-type (WT) and mPer1/mPer2 double knockout (DKO) mice. The ceramide concentration in WT mice was dramatically increased at Zeitgeber Time 9 (ZT9; 9 h after lights-on time) and ZT21 but no rhythmicity in ceramide expression was seen in DKO mice. Because ceramide can be generated by the hydrolysis of sphingomyelin via sphingomyelinase (SMase), or by ceramide synthase (CerS)-mediated synthesis, we assayed the expression patterns of ceramide-related genes using real-time PCR. CerS2 expression levels showed a biphasic pattern of expression in WT mice but no rhythmicity in DKO mice. While the neutral SMase (nSMase) and acidic SMase (aSMase) mRNA in WT mice were expressed in a circadian manner, the correlation between the expression levels of these SMases with times of day was weak in DKO mice. Collectively, our findings suggest that both SMases and CerS2 mRNA expression are regulated by the presence of mPer1/mPer2 circadian clock genes in vivo, and imply that ceramide may play a vital role in circadian rhythms and physiology.




Similar content being viewed by others
References
Weaver DR (1998) The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythm 13:100–112
Hirota T, Fukada Y (2004) Resetting mechanism of central and peripheral circadian clocks in mammals. Zool Sci 21:359–368
King DP, Takahashi JS (2000) Molecular genetics of circadian rhythms in mammals. Annu Rev Neurosci 23:713–742
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418:935–941
Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Science 280:1564–1569
King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS (1997) Positional cloning of the mouse circadian Clock gene. Cell 89:641–653
Lau P, Nixon SJ, Parton RG, Muscat GE (2004) RORα regulates the expression of genes involved in lipid homeostasis in skeletal muscle cells: Caveolin-3 and CPT-1 are direct targets of ROR. J Biol Chem 279:36828–36840
Inoue I, Shinoda Y, Ikeda M, Hayashi K, Kanazawa K, Nomura M, Matsunaga T, Xu H, Kawai S, Awata T, Komoda T, Katayama S (2005) CLOCK/BMAL1 is involved in lipid metabolism via transactivation of the peroxisome proliferator-activated receptor (PPAR) response element. J Atheroscler Thromb 12(3):169–174
Oishi K, Shirai H, Ishida N (2005) CLOCK is involved in the circadian transactivation of peroxisome-proliferator-activated receptor α (PPARα) in mice. Biochem J 386:575–581
Perry DK, Hannun YA (1998) The role of ceramide in cell signaling. Biochim Biophys Acta 1436:233–243
Obeid LM, Linardic CM, Karolak LA, Hannun Ya (1993) Programmed cell death induced by ceramide. Science 259:1769–1771
Igarashi Y (1997) Functional roles of sphingosine, sphingosine 1-phosphate, and methylsphingosines: in regard to membrane sphingolipid signaling pathways. J Biochem 122:1080–1087
Spiegel S, Merrill AH Jr (1996) Sphingolipid metabolism and cell growth regulation. FASEB J 10:1388–1397
Pettus BJ, Charles CE, Hannun YA (2002) Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta 1585:114–125
Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR (2001) Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron 30:525–536
Lee YS, Choi KM, Choi MH, Ji SY, Yoo JM, Lee YM, Hong JT, Yun YP, Yoo HS (2009) Simultaneous HPLC analysis of ceramide and dihydroceramide in human hairs. Arch Pharm Res 32:1795–1801
Kim KS, Lee KW, Baek IS, Lim CM, Krishnan V, Lee JK, Nestler EJ, Han PL (2008) Adenylyl cyclase-5 activity in the nucleus accumbens regulates anxiety-related behavior. J Neurochem 107:105–115
Durairaj G, Chaurasia P, Lahudkar S, Malik S, Shukla A, Bhaumik SR (2010) Regulation of chromatin assembly/disassembly by Rtt109p, a histone H3 Lys56-specific acetyltransferase, in Vivo. J Biol Chem 285:30472–30479
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Gachon F, Nagoshi E, Brown SA, Ripperger J, Schibler U (2004) The mammalian circadian timing system: from gene expression to physiology. Chromosoma 113:103–112
Lowrey PL, Takahashi JS (2004) Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet 5:407–441
Yagita K, Yamanaka I, Koinuma S, Shigeyoshi Y, Uchiyama Y (2009) Mini screening of kinase inhibitors affecting period-length of mammalian cellular circadian clock. Acta Histochem Cytochem 42:89–93
Hakomori S, Igarashi Y (1993) Gangliosides and glycosphingolipids as modulators of cell growth, adhesion, and transmembrane signaling. Adv Lipid Res 25:147–162
Hannun YA, Obeid LM (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 9:139–150
Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, Berdyshev EV, Haimovitz-Friedman A, Fuks Z, Kolesnick R (2010) Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell Signal 22:1300–1307
Deschl U, Cattley RC, Harada T, Kuettler K, Hailey JR, Hartig F, Leblanc B, Marsman DS, Shirai T (2001) Liver, gallbladder, and exocrine pancreas. In: Mohr U (ed) International classification of rodent tumors: the mouse, Springer-Verlag, 1st edn. Berlin, pp 59–85
Pewzner-Jung Y, Park H, Laviad EL, Silva LC, Lahiri S, Stiban J, Erez-Roman R, Brugger B, Sachsenheimer T, Wieland F, Prieto M, Merrill AH Jr, Futerman AH (2010) A critical role for ceramide synthase 2 in liver homeostasis: I. Alterations in lipid metabolic pathways. J Biol Chem 285:10902–10910
Bondy GS, Suzuki CA, Fernie SM, Armstrong CL, Hierlihy SL, Savard ME, Barker MG (1997) Toxicity of fumonisin B1 to B6C3F1 mice: a 14-day gavage study. Food Chem Toxicol 35:981–989
Sugiura M, Kono K, Liu H, Shimizugawa T, Minekura H, Spiegel S, Kohama T (2002) Ceramide kinase, a novel lipid kinase. Molecular cloning and functional characterization. J Biol Chem 277:23294–23300
Mitsutake S, Kim TJ, Inagaki Y, Kato M, Yamashita T, Igarashi Y (2004) Ceramide kinase is a mediator of calcium-dependent degranulation in mast cells. J Biol Chem 279:17570–17577
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MEST) to K. Bae (No. 2010-0016262).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jang, YS., Kang, YJ., Kim, TJ. et al. Temporal expression profiles of ceramide and ceramide-related genes in wild-type and mPer1/mPer2 double knockout mice. Mol Biol Rep 39, 4215–4221 (2012). https://doi.org/10.1007/s11033-011-1207-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1207-2