Abstract
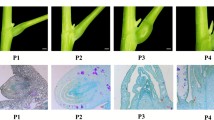
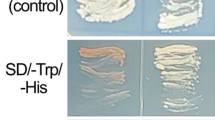
Rapid alkalinization factors (RALFs) are recently reported active peptide hormones and are considered to play important roles in plant development. We previously identified a differentially expressed cDNA fragment between cabbage flower buds of sterility lines and its maintainer line, which showed significant homology to Arabidopsis RALFL9. The novel RALF cDNA (BoRALF1) was isolated from broccoli flower buds by EST assembly. The open reading frame (ORF) comprises 240 bp, encoding a small putative preprotein of 79 amino acids (molecular weight of 8.72 kDa and a pI of 7.8), which contains the mature polypeptide at its C terminus. BoRALF1 shares 70.3% identity with Arabidopsis RALFL9, but has only moderate similarity with functionally characterized RALFs (ranging from 16.2% to 38.0%). BoRALF1 shows typical features of RALFs, including the 28-aa signal peptide, typical arrangement of four position conserved cysteines, the YIXY motif and a similar secondary structure. RT-PCR studies of different tissues and promoter-GUS fusions confirmed that BoRALF1 is expressed strictly in mature pollen grains and in the anther cells around the loculi. Based on in vivo transient assays, we found that BoRALF1 appears to be largely localized in the plasma membrane. Although the function of BoRALF1 remains to be determined, our experiments confirm the presence of RALF peptide in broccoli, and suggest it could have a role in anther or pollen development.




Similar content being viewed by others
References
Perez-Prat E, van Lookeren Campagne MM (2002) Hybrid seed production and the challenge of propagating male-sterile plants. Trends Plant Sci 7:199–203
Ma H (2005) Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol 56:393–434
Goldberg RB, Beals TP, Sanders PM (1993) Anther development: basic principles and practical applications. Plant Cell 5:1217–1229
Becker JD, Boavida LC, Carneiro J, Haury M, Feijo JA (2003) Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiol 133:713–725
Honys D, Twell D (2003) Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol 132:640–652
Pina C, Pinto F, Feijo JA, Becker JD (2005) Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiol 138:744–756
Matsubayashi Y, Sakagami Y (2006) Peptide hormones in plants. Annu Rev Plant Biol 57:649–674
Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM (1999) Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283:1911–1914
Pearce G, Moura DS, Stratmann J, Ryan CA Jr (2001) RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc Natl Acad Sci USA 98:12843–12847
Schopfer CR, Nasrallah ME, Nasrallah JB (1999) The male determinant of self-incompatibility in Brassica. Science 286:1697–1700
Olsen AN, Mundy J, Skriver K (2002) Peptomics, identification of novel cationic Arabidopsis peptides with conserved sequence motifs. In Silico Biol 2:441–451
Haruta M, Constabel CP (2003) Rapid alkalinization factors in poplar cell cultures. Peptide isolation, cDNA cloning, and differential expression in leaves and methyl jasmonate-treated cells. Plant Physiol 131:814–823
Germain H, Chevalier E, Caron S, Matton DP (2005) Characterization of five RALF-like genes from Solanum chacoense provides support for a developmental role in plants. Planta 220:447–454
Wu J, Kurten EL, Monshausen G, Hummel GM, Gilroy S, Baldwin IT (2007) NaRALF, a peptide signal essential for the regulation of root hair tip apoplastic pH in Nicotiana attenuata, is required for root hair development and plant growth in native soils. Plant J 52:877–890
Haruta M, Monshausen G, Gilroy S, Sussman MR (2008) A cytoplasmic Ca2 + functional assay for identifying and purifying endogenous cell signaling peptides in Arabidopsis seedlings: identification of AtRALF1 peptide. Biochemistry 47:6311–6321
Combier JP, Kuster H, Journet EP, Hohnjec N, Gamas P, Niebel A (2008) Evidence for the involvement in nodulation of the two small putative regulatory peptide-encoding genes MtRALFL1 and MtDVL1. Mol Plant Microbe Interact 21:1118–1127
Escobar NM, Haupt S, Thow G, Boevink P, Chapman S, Oparka K (2003) High-throughput viral expression of cDNA-green fluorescent protein fusions reveals novel subcellular addresses and identifies unique proteins that interact with plasmodesmata. Plant Cell 15:1507–1523
Scheer JM, Pearce G, Ryan CA (2005) LeRALF, a plant peptide that regulates root growth and development, specifically binds to 25 and 120 kDa cell surface membrane proteins of Lycopersicon peruvianum. Planta 221:667–674
Shiu SH, Bleecker AB (2001) Plant receptor-like kinase gene family: diversity, function, and signaling. Sci STKE 2001: RE22
Haerizadeh F, Wong CE, Bhalla PL, Gresshoff PM, Singh MB (2009) Genomic expression profiling of mature soybean (Glycine max) pollen. BMC Plant Biol 9:25
Lou P, Kang JG, Zhang GY, Bonnema G, Fang ZY, Wang XW (2007) Transcript profiling of a dominant male sterile mutant (Ms-cd1) in cabbage during flower bud development. Plant Sci 172:111–119
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27:297–300
Yu D, Chen C, Chen Z (2001) Evidence for an important role of WRKY DNA binding proteins in the regulation of NPR1 gene expression. Plant Cell 13:1527–1540
Chen W, Provart NJ, Glazebrook J, Katagiri F, Chang HS, Eulgem T, Mauch F, Luan S, Zou G, Whitham SA, Budworth PR, Tao Y, Xie Z, Chen X, Lam S, Kreps JA, Harper JF, Si-Ammour A, Mauch-Mani B, Heinlein M, Kobayashi K, Hohn T, Dangl JL, Wang X, Zhu T (2002) Expression profile matrix of Arabidopsis transcription factor genes suggests their putative functions in response to environmental stresses. Plant Cell 14:559–574
Rogers HJ, Bate N, Combe J, Sullivan J, Sweetman J, Swan C, Lonsdale DM, Twell D (2001) Functional analysis of cis-regulatory elements within the promoter of the tobacco late pollen gene g10. Plant Mol Biol 45:577–585
Matos JL, Fiori CS, Silva-Filho MC, Moura DS (2008) A conserved dibasic site is essential for correct processing of the peptide hormone AtRALF1 in Arabidopsis thaliana. FEBS Lett 582:3343–3347
Veenstra JA (2000) Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch Insect Biochem Physiol 43:49–63
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Sanders PM, Bui AQ, Weterings K, Mclntire KN, Hsu Y-C, Lee PY, Truong MT, Beals TP, Goldberg RB (1999) Anther development defects in Arabidopsis thaliana male sterile mutants. Sex Plant Reprod 11:297–322
Kang J, Zhang G, Bonnema G, Fang Z, Wang X (2008) Global analysis of gene expression in flower buds of Ms-cd1 Brassica oleracea conferring male sterility by using an Arabidopsis microarray. Plant Mol Biol 66:177–192
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: b-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Fan LM, Wang YF, Wang H, Wu WH (2001) In vitro Arabidopsis pollen germination and characterization of the inward potassium currents in Arabidopsis pollen grain protoplasts. J Exp Bot 52:1603–1614
Sakamoto W, Zaltsman A, Adam Z, Takahashi Y (2003) Coordinated regulation and complex formation of YELLOW VARIEGATED1 and YELLOW VARIEGATED2, chloroplastic FtsH metalloproteases involved in the repair cycle of photosystem II in Arabidopsis thylakoid membranes. Plant Cell 15:2843–2855
Ravanel S, Cherest H, Jabrin S, Grunwald D, Surdin-Kerjan Y, Douce R, Rebeille F (2001) Tetrahydrofolate biosynthesis in plants: Molecular and functional characterization of dihydrofolate synthetase and three isoforms of folylpolyglutamate synthetase in Arabidopsis thaliana. Proc Natl Acad Sci USA 98:15360–15365
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. 1
Structure and Phylogenetic analysis of BoRALF1. A, Nucleotide and deduced amino acid sequences of BoRALF1. The putative CAAT, TATA and TGAC boxes in the 5′-flanking region are underlined. The GTGA and AGAAA sequences, which are similar to the conserved sequence motifs of some other pollen-specific genes, are shown by open boxes. The instability motifs (ATTTA) in the 3′-UTR are shown as dotted lines. The mature peptide sequence of BoRALF1 is gray-shadowed. B, Sequences were aligned using the DNASTAR software package (version 7.1.0). The transmembrane regions are boxed; four conserved Cys residues constituting proposed disulfide bridges are marked with asterisks. The triangle indicates the predicted SP cleavage site and the black arrow shows the Arg-Arg motif. The dashed and dotted lines show the conserved motif (YIXY) and the C-terminal domain as designated by Ref. 14. C, Phylogenetic tree of RALFs. Amino acid sequences were aligned using ClustalW and the tree was inferred using MEGA-4.1 with the neighbor-joining method. Bootstrapped values are shown. RALFL21 was used as an outgroup as it was the most distantly related to other RALFs. Accession numbers are shown in parentheses, except for the following: LeRALF, ice plant, cotton, pea and wheat [9]. (JPG 3241 kb)
Rights and permissions
About this article
Cite this article
Zhang, Gy., Wu, J. & Wang, Xw. Cloning and expression analysis of a pollen preferential rapid alkalinization factor gene, BoRALF1, from broccoli flowers. Mol Biol Rep 37, 3273–3281 (2010). https://doi.org/10.1007/s11033-009-9912-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9912-9