Abstract
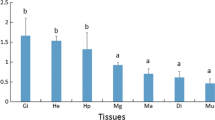
Heat shock protein 22 (HSP22) is an important member of small heat shock protein (sHSP) subfamily which plays a key role in the process of protecting cells, facilitating the folding of nascent peptides, and responding to stress. In the present study, the cDNA of HSP22 was cloned from Argopecten irradians (designated as AiHSP22) by rapid amplification cDNA end (RACE) based on the expressed sequence tags (ESTs). The full-length cDNA of AiHSP22 was of 1,112 bp, with an open reading frame of 588 bp encoding a polypeptide of 195 amino acids. The deduced amino acid sequence of AiHSP22 showed high similarity to previously identified HSP22s. The expression patterns of AiHSP22 mRNA in different tissues and in haemocytes of scallops exposed to Cd2+, Pb2+ or Cu2+ were investigated by real-time quantitative RT-PCR. The mRNA of AiHSP22 was constitutively expressed in all examined tissues, including haemocyte, muscle, kidney, gonad, gill and heart. The expression level in heart and muscle was higher than that in other tissues. The mRNA level of AiHSP22 in haemocytes was up-regulated after a 10 days exposure of scallops to Cu2+, Pb2+ and Cd2+. However, the expression of AiHSP22 did not increase linearly along with the rise of heavy metal concentration. Different concentrations of the same metal resulted in different effects on AiHSP22 expression. The sensitive response of AiHSP22 to Cu2+, Pb2+ and Cd2+ stress indicated that it could be developed as an indicator of exposure to heavy metals for the pollution monitoring programs in aquatic environment.





Similar content being viewed by others
References
Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22:631–677. doi:10.1146/annurev.ge.22.120188.003215
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579. doi:10.1038/381571a0
Feder ME, Hofmann GE (1999) Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol 61:243–282. doi:10.1146/annurev.physiol.61.1.243
Jolly C, Morimoto RI (1999) Stress and the cell nucleus: dynamics of gene expression and structural reorganization. Gene 7:261–270
Morimoto RI (1993) Cell in stress: transcriptional activation of heat shock genes. Science 259:1409–1410. doi:10.1126/science.8451637
Chowdary TK, Raman B, Ramakrishna T, Rao CM (2004) Mammalian Hsp22 is a heat-inducible small heat shock protein with chaperone-like activity. Biochem J 381:379–387. doi:10.1042/BJ20031958
Kohler HR, Triebskorn R, Stocker W, Kloetzel PM, Alberti G (1992) The 70 kD heat shock protein (hsp70) in soil invertebrates: a possible tool for monitoring environmental toxicants. Arch Environ Contam Toxicol 22:334–338. doi:10.1007/BF00212095
Sharp VA, Miller D, Bythell JC, Brown BE (1994) Expression of low molecular weight HSP70 related polypeptides from the symbiotic sea anemone Anemonia viridis Forskall in response to heat shock. J Exp Mar Biol Ecol 179:179–193. doi:10.1016/0022-0981(94)90113-9
De Pomerai D (1996) Heat-shock proteins as biomarkers of pollution. Hum Exp Toxicol 15:279–285. doi:10.1177/096032719601500401
Eckwert H, Kohler HR (1997) The indicative value of the hsp70 stress response as a marker for metal effects in Oniscus asellus (Isopoda) field populations: variability between populations from metal-polluted and uncontaminated sites. Appl Soil Toxicol 6:275–282. doi:10.1016/S0929-1393(97)00020-6
Lewis S, Handy RD, Cordi B, Billinghurst Z, Depledge MH (1999) Stress proteins (hsp’s): methods of detection and their use as an environmental biomarker. Ecotoxicology 8:351–368. doi:10.1023/A:1008982421299
Zhang F, Yang H (1999) Strategic and counter measures to resolve mass mortality problems of Chlamys farreri. Mark Sci 2:38–42
Mule MB, Lomte VS (1994) Effect of heavy metals (CuSO4 and HgCl2) on the oxygen consumption of the freshwater snail Thiara tuberculata. J Environ Biol 15:263–268
Baatrup E (1991) Structural and functional effects of heavy metals on the nervous system, including sense organs of fish. Comp Biochem Physiol C 100:253–257. doi:10.1016/0742-8413(91)90163-N
Jarup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182. doi:10.1093/bmb/ldg032
Gao Q, Song L, Ni D, Wu L, Zhang H, Chang Y (2007) cDNA cloning and mRNA expression of heat shock protein 90 gene in the haemocytes of Zhikong scallop Chlamys farreri. Comp Biochem Physiol B 147:704–715. doi:10.1016/j.cbpb.2007.04.010
Galloway TS, Depledge MH (2001) Immunotoxicity in invertebrates: measurement and ecotoxicological relevance. Ecotoxicology 10:5–23. doi:10.1023/A:1008939520263
Snyder MJ, Girvetz E, Mulder EP (2001) Induction of marine mollusc stress proteins by chemical or physical stress. Arch Environ Contam Toxicol 41:22–29. doi:10.1007/s002440010217
Boutet I, Tanguy A, Rousseau S, Auffret M, Moraga D (2003) Molecular identification and expression of heat shock cognate 70 (hsc70) and heat shock protein 70 (hsp70) genes in the Pacific oyster Crassostrea gigas. Cell Stress Chaperones 8:76–85. doi:10.1379/1466-1268(2003)8<76:MIAEOH>2.0.CO;2
Piano A, Valbonesi P, Fabbri E (2004) Expression of cytoprotective proteins, heat shock protein 70 and metallothioneins, in tissues of Ostrea edulis exposed to heat and heavy metals. Cell Stress Chaperones 9:134–142. doi:10.1379/483.1
Funes V, Alhama J, Navas JI, Lopez-Barea J, Peinado J (2006) Ecotoxicological effects of metal pollution in two mollusc species from the Spanish South Atlantic littoral. Environ Pollut 139:214–223. doi:10.1016/j.envpol.2005.05.016
Singer C, Zimmermann S, Sures B (2005) Induction of heat shock proteins (hsp70) in the zebra mussel (Dreissena polymorpha) following exposure to platinum group metals (platinum, palladium and rhodium): comparison with lead and cadmium exposures. Aquat Toxicol 75:65–75. doi:10.1016/j.aquatox.2005.07.004
Dondero F, Piacentini L, Banni M, Rebelo M, Burlando B, Viarengo A (2005) Quantitative PCR analysis of two molluscan metallothionein genes unveils differential expression and regulation. Gene 345:259–270. doi:10.1016/j.gene.2004.11.031
Song L, Wu L, Ni D, Chang Y (2006) The cDNA cloning and mRNA expression of heat shock protein 70 gene in the haemocytes of bay scallop (Argopecten irradians, Lamarck 1819) responding to bacteria challenge and naphthalin stress. Fish Shellfish Immunol 21:335–345
Song L, Xu W, Li C, Li H, Wu L (2006) Development of expressed sequence tags from the Bay Scallop Argopecten irradians. Mar Biotechnol 8:161–169. doi:10.1007/s10126-005-0126-4
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 25(4):402–408
Rainer B, Sun X, Robert R, Kelli J, Biederman Mark P (2001) HSP22, a new member of the small heat shock protein superfamily, interacts with mimic of phosphorylated HSP27 (3DHSP27). J Biol Chem 276:26753–26761. doi:10.1074/jbc.M103001200
Krahn MM, Myers MS, Burrows DG, Malins DC (1984) Determination of metabolites of xenobiotics in the bile of fish from polluted waterways. Xenobiotica 14:633–646
Coles JA, Farley SR, Pipe RK (1995) Alteration of the immune response of the common marine mussel Mytilus edulis resulting from exposure to cadmium. Dis Aquat Organ 22:59–65. doi:10.3354/dao022059
Pipe RK, Coles JA, Carissan FMM, Ramanathan K (1999) Copper induced immunomodulation in the marine mussel Mytilus edulis. Aquat Toxicol 46:43–54. doi:10.1016/S0166-445X(98)00114-3
Wang L, Song L, Ni D, Zhang H, Liu W (2009) Alteration of metallothionein mRNA in bay scallop Argopecten irradians under cadmium exposure and bacteria challenge. Comp Biochem Physiol C 149:50–57
Coles JA, Farley SR, Pipe RK (1994) Effects of fluoranthene on the immunocompetence of the common marine mussel Mytilus edulis. Aquat Toxicol 30:367–379. doi:10.1016/0166-445X(94)00051-4
Dyrynda EA, Law RJ, Dyrynda PEJ, Kelly CA, Pipe RK, Graham KL, Tatcliffe NA (1997) Modulations in cell-mediated immunity of the mussel Mytilus edulis following the Sea Empress oil spill. J Mar Biol 77:281–284
Elisabeth AD, Robin JL, Peter EJD, Carole AK, Richard KP, Norman AR (2000) Changes in immune parameters of natural mussel Mytilus edulis populations following a major oil spill. Mar Ecol Prog Ser 206:155–170. doi:10.3354/meps206155
Alvarez MR, Friedl FE, Hudson CM, O’Neill RL (1992) Effects of hypoxic and hyperoxic conditions on haemocyte activity and abiotic particle retention by the Eastern Oyster Crassostrea virginica. J Shellfish Res 11:383–386
Cruz-Rodriguez LA, Chu FL (2002) Heat-shock protein (hsp70) response in the eastern oyster, Crassostrea virginica, exposed to PAHs sorbed to suspended artificial clay particles and to suspended field contaminated sediments. Aquat Toxicol 60:157–168. doi:10.1016/S0166-445X(02)00008-5
Auffret M, Oubella R (1997) Haemocyte aggregation in the oyster Crassostrea gigas: in vitro measurement and experimental modulation by xenobiotics. Comp Biochem Physiol 118A:705–712. doi:10.1016/S0300-9629(97)00017-0
Baier-Anderson C, Anderson RS (2000) The effects of chlorothalonil on oyster haemocyte activation: phagocytosis, reduced pyridine nucleotides, and reactive oxygen species production. Environ Res 83:72–78. doi:10.1006/enrs.1999.4033
Law RJ (2000) The analysis of polycyclic aromatic hydrocarbons in marine samples. Int J Environ Pollut 13:262–283. doi:10.1504/IJEP.2000.002319
Law RJ, Hellou J (1999) Contamination of fish and shellfish following oil spill incidents. Environ Geosci 6:90–98. doi:10.1046/j.1526-0984.1999.08039.x
Beyersmann D, Hechtenberg S (1997) Cadmium, gene regulation, and cellular signalling in mammalian cells. Toxicol Appl Pharmacol 144:247–261. doi:10.1006/taap.1997.8125
Dundjerski J, Kovac T, Pavkovic N, Cvoro A, Matic G (2000) Glucocorticoid receptor-Hsp90 interaction in the liver cytosol of cadmium-intoxicated rats. Cell Biol Toxicol 16:375–383. doi:10.1023/A:1007600511094
Hermesz E, Abraham M, Nemcsok J (2001) Identification of two hsp90 genes in carp. Comp Biochem Physiol 129:397–407
Efremova SM, Margulis BA, Guzhova IV, Itskovich VB, Lauenroth S, Muller WE, Schroder HC (2003) Heat shock protein Hsp70 expression and DNA damage in Baikalian sponges exposed to model pollutants and wastewater from Baikalsk Pulp and Paper Plant. Aquat Toxicol 57:267–280. doi:10.1016/S0166-445X(01)00209-0
Radlowska M, Pempkowiak J (2002) Stress-70 as indicator of heavy metals accumulation in blue mussel Mytilus edulis. Environ Int 27:605–608. doi:10.1016/S0160-4120(01)00117-9
Andrew AS, Warren AJ, Barchowsky A, Temple KA, Klei L, Soucy NV, O’Hara KA, Hamilton JW (2003) Genomic and proteomic profiling of responses to toxic metals in human lung cells. Environ Health Perspect 111:825–835
Warchalowska-Sliwa E, Niklinska M, Gorlich A, Michailova P, Pyza E (2005) Heavy metal accumulation, heat shock protein expression and cytogenetic changes in Tetrix tenuicornis (L.) (Tetrigidae, Orthoptera) from polluted areas. Environ Pollut 133:373–381. doi:10.1016/j.envpol.2004.05.013
Ovelgonne JH, Souren JE, Wiegant FA, Van Wijk R (1995) Relationship between cadmium-induced expression of heat shock genes, inhibition of protein synthesis and cell death. Toxicology 99:19–30. doi:10.1016/0300-483X(94)02990-C
Wootton EC, Dyrynda EA, Ratcliffe NA (2003) Bivalve immunity: comparisons between the marine mussel (Mytilus edulis), the edible cockle (Cerastoderma edule) and the razor-shell (Ensis siliqua). Fish Shellfish Immunol 15:195–210. doi:10.1016/S1050-4648(02)00161-4
Pipe RK, Farley SR, Coles JA (1997) The separation and characterisation of haemocytes from the mussel Mytilus edulis. Cell Tissue Res 289:537–545. doi:10.1007/s004410050899
Lorenzon S, Francese M, Smith VJ, Ferrero EA (2001) Heavy metals affect the circulating haemocyte number in the shrimp Palaemon elegans. Fish Shellfish Immunol 11:459–472. doi:10.1006/fsim.2000.0321
Acknowledgments
The authors would like to thank all laboratory members for technical advice and helpful discussions. This research was supported by 863 High Technology Project (No. 2006AA10A402) from the Chinese Ministry of Science and Technology, and a commonweal project (No. nyhyzx07-047) from the Chinese Ministry of Agriculture, and grants (No. 30730070) from NSFC to Dr. Linsheng Song.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, L., Wang, L., Song, L. et al. The involvement of HSP22 from bay scallop Argopecten irradians in response to heavy metal stress. Mol Biol Rep 37, 1763–1771 (2010). https://doi.org/10.1007/s11033-009-9603-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-009-9603-6