Abstract
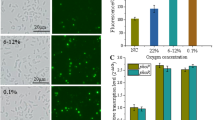
Glutaredoxin (Grx), also known as thioltransferase (TTase), is an enzyme that catalyzes the reduction of a variety of disulfide compounds, including protein disulfides, in the presence of reduced glutathione. A second gene encoding Grx (Grx2) was cloned from the chromosomal DNA of the fission yeast Schizosaccharomyces pombe. The determined DNA sequence contains 1645 bp which is able to encode a polypeptide of 110 amino acids with a molecular mass of 12.2 kDa. The genomic DNA consists of 4 exons and 3 introns. The isolated gene was found to produce functional glutaredoxin that could accelerate the growth of the fission yeast, and is highly expressed at the mid- and late exponential phases. Aluminum, cadmium and hydrogen peroxide marginally enhanced the synthesis of β-galactosidase from the Grx2-lacZ fusion gene. Shifts to lower concentrations (0.2, 0.4 or 0.8%) of d-glucose significantly enhanced the synthesis of β-galactosidase from the Grx2-lacZ fusion gene. And shifts to sucrose (0.2, 0.4, 0.8 or 1.6%) as a sole carbon source markedly enhanced the synthesis of β-galactosidase from the Grx2-lacZ fusion gene, the degree of which was inversely dependent on concentration. However, nonfermentable carbon sources reduced the expression of the Grx2 gene due to their growth arrest. The transcription factor Pap1 is not involved in the basal expression and induction of the Grx2 gene. The Grx2 protein was subcellularly localized in the nucleus of the yeast cells. Our results indicate that the Grx2 protein, located in the nucleus, is linked with the yeast growth, and that the gene is regulated by carbon sources.
Similar content being viewed by others
Abbreviations
- GFP:
-
green fluorescent protein
- Grx:
-
glutaredoxin
- HED:
-
2-hydroxyethyl disulfide
- ONPG:
-
o-nitrophenyl β-d-galactopyranoside
- PCR:
-
polymerase chain reaction
References
JH Bushweller F Áslund K Wuthrich A Holmgren (1992) Biochem. 31 9288–9293 Occurrence Handle1:CAS:528:DyaK38Xmt1yqsr8%3D
A Holmgren F Áslund (1995) Methods Enzymol. 252 283–292 Occurrence Handle1:CAS:528:DyaK2MXptFOrtLY%3D Occurrence Handle7476363
JJ Mieyal SA Gravina PA Mieyal U Srinivasan DW Starke (1995) NoChapterTitle L Packer E Cadenas (Eds) Biothiols in Health and Disease Marcel Dekker, Inc. New York 305–372
F Qiao K Xing MF Lou (2000) Exp. Eye Res. 70 745–753 Occurrence Handle1:CAS:528:DC%2BD3cXjvVSktL0%3D Occurrence Handle10843779
LM Grimm MW Collison RA Fisher JA Thomas (1985) Biochim. Biophys. Acta 844 50–54 Occurrence Handle1:CAS:528:DyaL2MXhtFSnsLk%3D Occurrence Handle3967051
J Wang ES Boja W Tan E Tekel H Fales SM English JJ Mieyal PB Chock (2001) J. Biol. Chem. 276 47763–47766 Occurrence Handle1:CAS:528:DC%2BD38XkvFWh Occurrence Handle11684673
D Daily A Vlamis-Gardikas D Offen L Mittelman E Melamed A Holmgren A Barzilai (2001) J. Biol. Chem. 276 1335–1344 Occurrence Handle1:CAS:528:DC%2BD3MXmtV2jtw%3D%3D Occurrence Handle11035035
S Bandyopadhyay DW Starke JJ Mieyal RM Gronostajski (1998) J. Biol. Chem. 273 392–397 Occurrence Handle1:CAS:528:DyaK1cXjvFWnsg%3D%3D Occurrence Handle9417094
WC Barrett JP DeGnore S Konig HM Fales YF Keng ZY Zhang MB Yim PB Chock (1999) Biochem. 38 6699–6705 Occurrence Handle1:CAS:528:DyaK1MXis1yksL8%3D
T Nakamura T Ohno K Hirota A Nishiyama H Nakamura H Wada J Yodoi (1999) Free Radic. Res. 31 357–365 Occurrence Handle1:CAS:528:DyaK1MXmslGjsLc%3D Occurrence Handle10517541
C-H Jung JA Thomas (1996) Arch. Biochem. Biophys. 335 61–72 Occurrence Handle1:CAS:528:DyaK28XmvVOls7w%3D Occurrence Handle8914835
S Luikenhuis G Perrone IW Dawes CM Grant (1998) Mol. Biol. Cell 9 1081–1091 Occurrence Handle1:CAS:528:DyaK1cXivFKksbw%3D Occurrence Handle9571241
CA Chrestensen CB Eckman DW Starke JJ Mieyal (1995) FEBS Lett. 374 25–28 Occurrence Handle1:CAS:528:DyaK2MXovVynsrY%3D Occurrence Handle7589505
JJ Song YJ Lee (2003) Biochem. J. 373 845–853 Occurrence Handle1:CAS:528:DC%2BD3sXlslOgsrg%3D Occurrence Handle12723971
M Okuda N Inoue H Azumi T Seno Y Sumi K Hirata S Kawashima Y Hayashi H Itoh J Yodoi M Yokoyama (2001) Arterioscler. Thromb. Vasc. Biol. 21 1483–1487 Occurrence Handle1:CAS:528:DC%2BD3MXntVGku7Y%3D Occurrence Handle11557676
CL White AS Weisberg B Moss (2000) J. Virol. 74 9175–9183 Occurrence Handle1:CAS:528:DC%2BD3cXmslymt7Y%3D Occurrence Handle10982364
DA Davis FM Newcomb DW Starke DE Ott JJ Mieyal R Yarchoan (1997) J. Biol. Chem. 272 25935–25940 Occurrence Handle1:CAS:528:DyaK2sXms1Ggtb8%3D Occurrence Handle9325327
MT Rodríguez-Manzaneque J Ros E Cabiscol A Sorribas E Herrero (1999) Mol. Cell. Biol. 19 8180–8190 Occurrence Handle10567543
CM Grant S Liukenhuis A Beckhouse M Soderbergh IW Dawes (2000) Biochim. Biophys. Acta 1490 33–42 Occurrence Handle1:CAS:528:DC%2BD3cXhsFCis7k%3D Occurrence Handle10786615
JR Pedrajas P Porras E Martinez-Galistes CA Padilla A Miranda-Vizuette JA Barcena (2002) Biochem. J. 364 617–623 Occurrence Handle1:CAS:528:DC%2BD38XltF2it7o%3D Occurrence Handle11958675
H-G Kim E-H Park C-J Lim (1998) Mol. Cells 8 431–437 Occurrence Handle1:CAS:528:DyaK1cXlvFWqu70%3D Occurrence Handle9749530
H-G Kim E-H Park C-J Lim (1999) J. Biochem. Mol. Biol. 32 535–540 Occurrence Handle1:CAS:528:DyaK1MXnvFKmtLs%3D
Y-W Cho H-G Kim E-H Park JA Fuchs C-J Lim (2000) Biochim. Biophys. Acta 1517 171–175 Occurrence Handle1:CAS:528:DC%2BD3cXoslGmsLk%3D Occurrence Handle11118633
C-J Lim Y-W Cho S-M Hong H-W Lim E-H Park (2003) Mol. Cells 16 123–127 Occurrence Handle1:CAS:528:DC%2BD3sXnvFOgtLw%3D Occurrence Handle14503856
AM Myers A Tzagoloff DM Kinney CJ Lusty (1986) Gene 45 299–310 Occurrence Handle1:CAS:528:DyaL2sXksV2kug%3D%3D Occurrence Handle3026915
SN Cohen AC Chang L Hsu (1972) Proc. Natl. Acad. Sci. USA 69 2110–2114 Occurrence Handle1:CAS:528:DyaE38XltFWgtLo%3D Occurrence Handle4559594
A Holmgren (1979) J. Biol. Chem. 254 3664–3671 Occurrence Handle1:CAS:528:DyaE1MXktlCkurs%3D Occurrence Handle372193
L Guarente (1983) Methods Enzymol. 101 181–191 Occurrence Handle1:CAS:528:DyaL2cXhvVOhsA%3D%3D Occurrence Handle6310321
MM Bradford (1976) Anal. Biochem. 72 248–254 Occurrence Handle1:CAS:528:DyaE28XksVehtrY%3D Occurrence Handle942051
Sambrook J, Fritsch EF & Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
H-G Kim Y-W Cho E-H Park S-S Park K-S Ahn C-J Lim (1999) Mol. Cells 9 668–672 Occurrence Handle1:CAS:528:DC%2BD3cXltFChsA%3D%3D Occurrence Handle10672936
A Miranda-Vizuete JR Pedrajas AE Damdimopoulos G Spyrou (1999) DNA Seq. 10 179–182 Occurrence Handle1:CAS:528:DC%2BD3cXivVKksr4%3D Occurrence Handle10647820
J Ehrhart M Gluck J Mieyal GD Zeevalk (2002) Parkinsonism Relat. Disord. 8 395–400 Occurrence Handle12217626
MR Fernando H Sumimoto H Nanri S Kawabata S Iwanaga S Minakami Y Fukumaki K Takeshige (1994) Biochim. Biophys. Acta 1218 229–231 Occurrence Handle1:CAS:528:DyaK2cXlslersrY%3D Occurrence Handle8018729
YF Yang ZR Gan WW Wells (1989) Gene 83 339–346 Occurrence Handle1:CAS:528:DyaK3cXktFyrtLw%3D Occurrence Handle2583530
IA Papayannopoulos ZR Gan WW Wells K Biemann (1989) Biochem. Biophys. Res. Commun. 159 1448–1454 Occurrence Handle1:CAS:528:DyaL1MXktF2ls7o%3D Occurrence Handle2930571
S Hopper RS Johnson JE Vath K Biemann (1989) J. Biol. Chem. 264 20438–20447 Occurrence Handle1:CAS:528:DyaK3cXltVyitA%3D%3D Occurrence Handle2684977
IA Cotgreave RC Gerdes (1998) Biochem. Biophys. Res. Commun. 242 1–9 Occurrence Handle1:CAS:528:DyaK1cXjtl2qtA%3D%3D Occurrence Handle9439600
HM Hassan I Fridovich (1979) Arch. Biochem. Biophys. 196 385–395 Occurrence Handle1:CAS:528:DyaE1MXltFOqs78%3D Occurrence Handle225995
JM Thevelein L Cauwenberg S Colombo JH DeWinde M Donation F Dumortier L Kraakman K Lemaire P Ma D Nauwelaers F Rolland A Teunissen Dijck P Van M Versele S Wera J Winderickx (2000) Enzyme Microb. Technol. 26 819–825 Occurrence Handle1:CAS:528:DC%2BD3cXktFSjur8%3D Occurrence Handle10862891
MJ Kim C-J Lim D Kim (2002) J. Biochem. Mol. Biol. 35 409–413 Occurrence Handle1:CAS:528:DC%2BD38XmtF2ntbo%3D Occurrence Handle12297001
M Hiesinger S Roth E Meissner H-J Schüller (2001) Curr. Genet. 39 68–76 Occurrence Handle1:CAS:528:DC%2BD3MXivFaisrg%3D Occurrence Handle11405098
K Walther H-J Schüller (2001) Microbiol. 147 2037–2044 Occurrence Handle1:CAS:528:DC%2BD3MXmt12qu70%3D
WM Toone S Kuge M Samuels BA Morgan T Toda N Jones (1998) Genes Dev. 12 23042–23049
AN Nguyen A Lee W Place K Shiozaki (2000) Mol. Biol. Cell 11 1169–1181 Occurrence Handle1:CAS:528:DC%2BD3cXlt1Wlu7o%3D Occurrence Handle10749922
Z Gan WW Wells (1988) J. Biol. Chem. 263 9050–9054 Occurrence Handle1:CAS:528:DyaL1cXks12htbo%3D Occurrence Handle2454232
M Lundberg C Johansson J Chandra M Enoksson G Jacobsson J Ljung M Johansson A Holmgren (2001) J. Biol. Chem. 276 26269–26275 Occurrence Handle1:CAS:528:DC%2BD3MXlsVKnsb8%3D Occurrence Handle11297543
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, HG., Kim, JH., Kim, BC. et al. Carbon source-dependent regulation of a second gene encoding glutaredoxin from the fission yeast Schizosaccharomyces pombe . Mol Biol Rep 32, 15–24 (2005). https://doi.org/10.1007/s11033-004-3213-0
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11033-004-3213-0