Abstract
Previous study showed that two alcohol dehydrogenase genes (ADHs) were significantly upregulated in roots of Actinidia deliciosa after treatment with waterlogging using Illumina sequencing technology. Those two ADH genes, named AdADH1 and AdADH2, were isolated from A. deliciosa in this study. QRT-PCR analysis showed that AdADH1 and AdADH2 expression levels were significantly increased in A. deliciosa after treatment with waterlogging, NaCl, 4 °C, and heat stresses, but not ABA and drought stresses. The changes of AdADH1 and AdADH2 expression levels during waterlogging were much higher than those during other stresses. We examined the response to abiotic stresses in transgenic Arabidopsis lines in which the A. deliciosa AdADH1 and AdADH2 genes were introduced under the control of a constitutive promoter, respectively. Overexpression of A. deliciosa AdADH1 or AdADH2 in Arabidopsis could enhance waterlogging tolerance at five week old seedlings. However, the function of AdADH1 for waterlogging resistance was much better than that of AdADH2. Arabidopsis overexpressing AdADH1 gene could enhance the resistance to cold stress at five week old seedlings, but AdADH2 could not. Overexpression of AdADH1 gene enhances the resistance to salt stress at the stage of seed germination and in seedlings, and overexpression of AdADH2 gene enhances the resistance to salinity stress at the stage of seed germination but not in seedlings. However, transgenic Arabidopsis overexpressing AdADH1 or AdADH2 could not enhance to the osmotic stress at the stage of seed germination and in seedlings. These results indicated that A. deliciosa AdADH1 plays an important role in resistance to waterlogging, salinity, and cold stresses, but not drought stress, and AdADH2 plays an important role in resistance to waterlogging and salinity stresses, but not cold and drought stresses.





Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- ADH:
-
Alcohol dehydrogenase
- CaMV:
-
Cauliflower mosaic virus
- HRE1/2 :
-
Hypoxia responsive ERF1/2
- IBA:
-
Indole-3-butytric acid
- MS:
-
Murashige and Skoog
- PDC:
-
Pyruvate decarboxylase
- qRT-PCR:
-
Real time quantitative RT-PCR
- WT:
-
Wild type
References
Bruxelles CL, Peacock WJ, Dennis ES, Dolferus R (1996) Abscisic acid induces the alcohol dehydrogenase gene in Arabidopsis. Plant Physiol 111:381–391
Cai BH, Zhang JY, Gao ZH, Qu SC, Tong ZG, Mi L, Qiao YS, Zhang Z (2008) An improved method for isolation of total RNA from the leavesof Fragaria spp. Jiangsu J Agric Sci 24:875–877
Christianson JA, Llewellyn DJ, Dennis ES, Wilson IW (2010) Global gene expression responses to waterlogging in roots and leaves of cotton (Gossypium hirsutum L.) Plant Cell Physiol 51:21–37
Christie PJ, Hahn M, Walbot V (1991) Low-temperature accumulation of alcohol dehydrogenase-1 mRNA and protein activity in maize and rice. Plant Physiol 95:699–706
Chung HJ, Ferl RJ (1999) Arabidopsis alcohol dehydrogenase expression in both shoots and roots is conditioned by root growth environment. Plant Physiol 121(2):429–436
Clough SJ, Bent AF (1998) Floral dip: a simplified method for agrobacterium -mediated transformation of Arabidopsis thaliana. Plant J 16(6):735–743
Conley TR, Peng H-P, Shih M-C (1999) Mutations affecting induction of glycolytic and fermentative genes during germination and environmental stresses in Arabidopsis. Plant Physiol 119:599–607
Davik J, Koehler G, From B, Torp T, Rohloff J, Eidem P, Wilson RC, Sønsteby A, Randall SK, Alsheikh M (2012) Dehydrin, alcohol dehydrogenase, and central metabolite levels are associated with cold tolerance in diploid strawberry (Fragaria spp.) Planta 237:265–277
Diab AA, Kantety RV, Ozturk NZ, Benscher D, Nachit MM, Sorrells ME (2008) Drought - inducible genes and differentially expressed sequence tags associated with components of drought tolerance in durum wheat. Sci Res Essay 3:9–26
Dolferus R, Jacobs M, Peacock WJ, Dennis ES (1994) Differential interactions of promoter elements in stress responses of the Arabidopsis Adh gene. Plant Physiol 105:1075–1087
Fukuda T, Yokoyama J, Nakamura T, Song I-J, Ito T, Ochiai T, Kanno A, Kameya T, Maki M (2005) Molecular phylogeny and evolution of alcohol dehydrogenase (Adh) genes in legumes. BMC Plant Biol 5:6
Garabagi F, Duns G, Strommer J (2005) Selective recruitment of Adh genes for distinct enzymatic functions in Petunia hybrida. Plant Mol Biol 58:283–294
Gibbs D, Lee S, Isa N, Gramuglia S, Fukao T, Bassel G, Correia C, Corbineau F, Theodoulou F, Bailey-Serres J (2011) Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479:415–418
Harberd N, Edwards K (1982) The effect of a mutation causing alcohol dehydrogenase deficiency on flooding tolerance in barley. New Phytol 90:631–644
Ismond K, Dolferus R, Pauw M, Dennis E, Good A (2003) Enhanced low oxygen survival in arabidopsis through increased metabolic flux in the fermentative pathway. Plant Physiol 132:1292–1302
Jackson MB, Colmer TD (2005) Response and adaptation by plants to flooding stress. Ann Bot 96:501–505
Jacobs M, Dolferus R, Van Den Bosshe V (1988) Isolation and biochemical analysis of ethyl methyl sulfonate induced alcohol dehydrogenase null mutants of Arabidopsis thaliana (L.) Heynth. Biochem Genet 26:102–112
Jarillo JA, Leyva A, Salinas J, M-Z M (1993) Low temperature induces the accumulation of alcohol dehydrogenase mRNA in Arabidopsis thaliana. Plant Physiol 101:833–837
Johnson JR, Cobb BC, Drew MC (1994) Hypoxic induction of anoxia tolerance in roots of Adh1 null Zea mays L. Plant Physiol 105:61–67
Kürsteiner O, Dupuis I, Kuhlemeier C (2003) The pyruvate decarboxylase1 gene of Arabidopsis is required during anoxia but not other environmental stresses. Plant Physiol 132:968–978
Kato-Noguchi H (1999) Flooding induction of alcohol dehydrogenase in shoots and roots of barley. Acta Physiol Plant 21:17–20
Kato-Noguchi H, Yasuda Y (2007) Effect of low temperature on ethanolic fermentation in rice seedlings. J Plant Physiol 164:1013–1018
Komatsu S, Kobayashi Y, Nishizawa K, Nanjo Y, Furukawa K (2010a) Comparative proteomics analysis of differentially expressed proteins in soybean cell wall during flooding stress. Amino Acids 39:1435–1449
Komatsu S, Sugimoto T, Hoshino T, Nanjo Y, Furukawa K (2010b) Identification of flooding stress responsible cascades in root and hypocotyl of soybean using proteome analysis. Amino Acids 38:729–738
Komatsu S, Thibaut D, Hiraga S, Kato M, Chiba M, Hashiguchi A, Tougou M, Shimamura S, Yasue H (2011) Characterization of a novel flooding stress-responsive alcohol dehydrogenase expressed in soybean roots. Plant Mol Biol 77:309–322
Komatsu S, Yamamoto R, Nanjo Y, Mikami Y, Yunokawa H, Sakata K (2009) A comprehensive analysis of the soybean genes and proteins expressed under flooding stress using transcriptome and proteome techniques. J Proteome Res 8:4766–4778
Kosmacz M, Parlanti S, SchwarzlÄNder M, Kragler F, Licausi F, Van Dongen JT (2015) The stability and nuclear localization of the transcription factor RAP2.12 are dynamically regulated by oxygen concentration. Plant Cell Environ 38:1094–1103
Liang Y, Yan J-p, Tan X-l (2012) Effects of salt-stress on expression of ADH3 and ALDH in radicle of rice (Oryza sativa). Hubei Agric Sci 51:2870–2872
Licausi F (2011) Regulation of the molecular response to oxygen limitations in plants. New Phytol 190:550–555
Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LACJ, Perata P, van Dongen JT (2011) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479:419–422
Licausi F, Van Dongen JT, Giuntoli B, Novi G, Santaniello A, Geigenberger P, Perata P (2010) HRE1 and HRE2, two hypoxia-inducible ethylene response factors, affect anaerobic responses in Arabidopsis thaliana. Plant J 62:302–315
Lin K-H, Lin C-H, Chan M-T, Lo H-F (2010) Identification of flooding-response genes in eggplant roots by suppression subtractive hybridization. Plant Mol Biol Report 28:212–221
Liu Z, Adams KL (2007) Expression partitioning between genes duplicated by polyploidy under abiotic stress and during organ development. Curr Biol 17:1669–1674
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Matsumura H, Takano T, Takeda G, Uchimiya H (1998) Adh1 is transcriptionally active but its translational product is reduced in a rad mutant of rice (Oryza sativa L.), which is vulnerable to submergence stress. Theor Appl Genet 97:1197–1203
Min T, Yin X-r, Shi Y-n, Luo Z-r, Yao Y-c, Grierson D, Ferguson IB, Chen K-s (2012) Ethylene-responsive transcription factors interact with promoters of ADH and PDC involved in persimmon (Diospyros kaki) fruit de-astringency. J Exp Bot 63:6393–6405
Nanjo Y, Skultety L, Ashraf Y, Komatsu S (2010) Comparative proteomic analysis of early- stage soybean seedlings responses to flooding by using gel and gel-free techniques. J Proteome Res 9:3989–4002
Narsai R, Whelan J (2013) How unique is the low oxygen response? An analysis of the anaerobic response during germination and comparison with abiotic stress in rice and Arabidopsis. Front Plant Sci 4:349
Peters JS, Frenkel C (2004) Relationship between alcohol dehydrogenase activity and low-temperature in two maize genotypes, Silverado F1 and Adh1 − Adh2 − doubly null. Plant Physiol Biochem 42:841–846
Rahman M, Grover A, Peacock W, Dennis E, Ellis M (2001) Effects of manipulation of pyruvate decarboxylase and alcohol dehydrogenase levels on the submergence tolerance of rice. Aust J Plant Physiol 28:1231–1241
Rossmann M, Moras D, Olsen K (1974) Chemical and biological evolution of a nucleotide-binding protein. Nature 250:194–199
Savé R, Serrano L (1986) Some physiological and growth responses of kiwi fruit (Actinidia chinensis) to flooding. Physiol Plant 66(1):75–78
Senthil-Kumar M, Hema R, Suryachandra TR, Ramegowda HV, Gopalakrishna R, Rama N, Udayakumar M, Mysore KS (2010) Functional characterization of three water deficit stress-induced genes in tobacco and Arabidopsis: an approach based on gene down regulation. Plant Physiol Biochem 48:35–44
Stewart A, Mccarrison AM (1992) The role of phytophthora cryptogea and waterlogging in root rot kiwifruit. J Phytopathol 136(3):194–198
Strommer J (2011) The plant ADH gene family. Plant J 66:128–142
Tacconi G, Paltrinieri S, Mejia JF, Fuentealba SP, Bertaccini A, Tosi L, Giacopini A, Mazzucchi U, Favaron F, Sella L, Bertaiola F (2015) Vine decline in kiwifruit: climate change and effect on waterlogging and phytophthora in north italy. Acta Hortic 1096:93–97
Tamang BG, Magliozzi JO, Maroof MAS, Fukao T (2014) Physiological and transcriptomic characterization of submergence and reoxygenation responses in soybean seedlings. Plant Cell Environ 37:2350–2360
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tesniere C (2005) Effects of genetic manipulation of alcohol dehydrogenase levels on the response to stress and the synthesis of secondary metabolites in grapevine leaves. J Exp Bot 57:91–99
Tougou M, Hashiguchi A, Yukawa K, Nanjo Y, Hiraga S, Nakamura T, Nishizawa K, Komatsu S (2012) Responses to flooding stress in soybean seedlings with the alcohol dehydrogenase transgene. Plant Biotechnol 29:301–305
Voesenek LACJ, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytol 206:57–73
Yin D, Chen S, Chen F, Guan Z, Fang W (2009) Morphological and physiological responses of two chrysanthemum cultivars differing in their tolerance to waterlogging. Environ Exp Bot 67:87–93
Yin D, Ni D, Song L, Zhang Z (2013) Isolation of an alcohol dehydrogenase cDNA from and characterization of its expression in chrysanthemum under waterlogging. Plant Sci 212:48–54
Yin X-R, Allan AC, Xu Q, Burdon J, Dejnoprat S, Chen K-S, Ferguson IB (2012) Differential expression of kiwifruit ERF genes in response to postharvest abiotic stress. Postharvest Biol Technol 66:1–7
Zhang J-Y, Huang S-N, Mo Z-H, Xuan J-P, Jia X-D, Wang G, Guo Z-R (2015a) De novo transcriptome sequencing and comparative analysis of differentially expressed genes in kiwifruit under waterlogging stress. Mol Breed 35:208
Zhang J-y, Qu S-c, Dong C, Gao Z-h, Qiao Y-s, Zhang Z (2010) Utility and construction of full-length cDNA library of Malus hupehensis post-introduced with salicylic acid. Acta Bot Boreal -Occident Sin 30:1527–1533
Zhang J, Wang G, Huang S, Xuan J, Jia X, Guo Z (2015b) Functions of alcohol dehydrogenase family in abiotic stress responses in plants. Chin Agric Sci Bull 31:246–250
Zhang JY, Qiao YS, Lv D, Gao ZH, Qu SC, Zhang Z (2012) Malus hupehensis NPR1 induces pathogenesis-related protein gene expression in transgenic tobacco. Plant Biol 14:46–56
Acknowledgements
This study was supported by the National Natural Science Foundation of China (NSFC) (31401854) and grants from the Natural Science Foundation of Jiangsu Province (G rants No BK20140760).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplemental Table 1
Primers used for this paper (DOCX 19 kb)
Supplemental Fig. 1
Derived amino acid sequences alignment of AdADH1 and AdADH2 with proteins from Oryza sativa (OsADH1, Genbank accession no. AAF34414; OsADH2, Genbank accession no. AAF34412) and Vitis vinifera (VvADH1, Genbank accession no. NP_001268079; VvADH2, Genbank accession no. NP_001268083). Dots indicate that amino acids sequences are conserved. The major functional domains are indicated, along with the binding sites of catalytic (solid triangle) and structural (solid diamond) Zn ions. (GIF 55 kb)
Supplemental Fig. 2
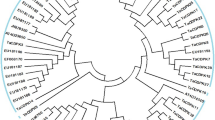
Phylogenetic analyses of ADH proteins. The deduced amino acids sequences were obtained from the NCBI, the species and the accession numbers are indicated. The phylogenetic tree was conducted using the MEGA5 based on the full-length amino acid sequences. Bootstrap analysis with 1000 replicates was performed to obtain confidence levels for the branches of the tree. (GIF 30 kb)
Supplemental Fig. 3
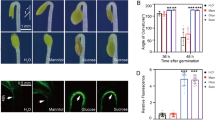
Comparison of germination rates and root length between AdADH1 transgenic (ADH1–1, ADH1–2, ADH1–3) and wild-type (WT) lines under NaCl stresses. A photograph was taken 10 d after seed germination of WT and transgenic Arabidopsis plants in MS medium containing various concentrations of NaCl (a). Germination rates (c) in WT and transgenic plants grown in MS (control) or MS supplemented with various concentrations of NaCl for 7 d. Germination rates results are presented as mean ± SD from three independent experiments (at least 50 seeds of each line were grown for each experiment). Four day old seedlings of WT and transgenic seedlings which were germinated just from MS agar medium were transferred to MS medium containing different concentrations of NaCl. The phenotypic (b) and root length (c) were measured after 7 days. The experiments were carried out on three replicates of 30 seedlings. The results from one set of experiments are shown. Double asterisks indicate significant differences compared with WT lines values (P < 0.01). (GIF 207 kb)
Supplemental Fig. 4
Comparison of germination rates and root length between AdADH2 transgenic (ADH2–1, ADH2–2, ADH2–3) and wild-type (WT) lines under NaCl stresses. A photograph was taken 10 d after seed germination of WT and transgenic Arabidopsis plants in MS medium containing various concentrations of NaCl (a). Germination rates (b) in WT and transgenic plants grown in MS (control) or MS supplemented with various concentrations of NaCl for 7 d. Germination rates results are presented as mean ± SD from three independent experiments (at least 50 seeds of each line were grown for each experiment). Four day old seedlings of WT and transgenic seedlings which were germinated just from MS agar medium were transferred to MS medium containing different concentration of NaCl. The phenotypic (c) and rooting length (d) were measured after 7 days. The experiments were carried out on three replicates of 30 seedlings. The results from one set of experiments are shown. Double asterisks indicate significant differences compared with WT lines values (P < 0.01). (GIF 200 kb)
Supplemental Fig. 5
Comparison of germination rates and root length between AdADH1 transgenic (ADH1–1, ADH1–2, ADH1–3) and wild-type (WT) lines under mannitol stresses. A photograph was taken after 10 d of seed germination of WT and transgenic Arabidopsis plants in MS medium containing various concentrations of mannitol (a). Germination rates (b) in WT and transgenic plants grown in MS (control) or MS supplemented with various concentrations of mannitol for 7 d. Germination rates results are presented as mean ± SD from three independent experiments (at least 50 seeds of each line were grown for each experiment). Four day old seedlings of WT and transgenic seedlings which were germinated just from MS agar medium were transferred to MS medium containing 0.15 M mannitol. The phenotypic (c) and rooting length (d) were measured after 7 days. The experiments were carried out on three replicates of 30 seedlings. The results from one set of experiments are shown. (GIF 119 kb)
Supplemental Fig. 6
Comparison of germination rates and root length between AdADH2 transgenic (ADH2–1, ADH2–2, ADH2–3) and wild-type (WT) lines under mannitol stresses. A photograph was taken after 10 d of seed germination of WT and transgenic Arabidopsis plants in MS medium containing various concentrations of mannitol (a). Germination rates (c) in WT and transgenic plants grown in MS (control) or MS supplemented with various concentrations of mannitol for 7 d. Germination rates results are presented as mean ± SD from three independent experiments (at least 50 seeds of each line were grown for each experiment). Four day old seedlings of WT and transgenic seedlings which were germinated just from MS agar medium were transferred to MS medium containing 0.15 M mannitol. The phenotypic (b) and rooting length (d) were measured after 7 days. The experiments were carried out on three replicates of 30 seedlings. The results from one set of experiments are shown. (GIF 115 kb)
Rights and permissions
About this article
Cite this article
Zhang, JY., Huang, SN., Chen, YH. et al. Identification and characterization of two waterlogging responsive alcohol dehydrogenase genes (AdADH1 and AdADH2) in Actinidia deliciosa . Mol Breeding 37, 52 (2017). https://doi.org/10.1007/s11032-017-0653-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11032-017-0653-5