Abstract
A novel series of benzenesulfonamide substituted spirothiazolidinone derivatives (3a–j) were synthesized, characterized and evaluated for their antiviral activity. The spirocyclic compounds were prepared by the condensation of 4-(aminosulfonyl)-2-methoxybenzohydrazide, appropriate cyclic ketones and 2-mercaptopropionic acid in a one-pot reaction. The structures of the new compounds were established by IR, 1H NMR, 13C NMR (APT), and elemental analysis. The new compounds were evaluated in vitro antiviral activity against influenza A/H1N1, A/H3N2 and B viruses, as well as herpes simplex virus type 1 (HSV-1), respiratory syncytial virus (RSV) and yellow fever virus (YFV). Two derivatives bearing propyl (3d) and tert-butyl (3e) substituents at position 8 of the spiro ring exhibited activity against influenza A/H1N1 virus with EC50 values in the range of 35–45 µM and no cytotoxicity at 100 μM, the highest concentration tested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Influenza A and B viruses are highly contagious human respiratory pathogens causing annual epidemics with high medical and socioeconomical burden [1]. Influenza A viruses are classified into different A/HxNx subtypes on the basis of two surface glycoproteins, hemagglutinin (HA; 18 known subtypes) and neuraminidase (NA; 11 known subtypes) [2]. The current strains of seasonal influenza A virus belong to subtype A/H1N1 [specifically, A(H1N1)pdm09, that entered the human population during the 2009 pandemic] and A/H3N2. For influenza B virus, the strains are divided in two phylogenetic lineages named B/Victoria and B/Yamagata [3]. Within the viral replication cycle, HA is required for virus attachment and entry, while NA mediates release of the virus as well as its penetration through mucus [4].
Since the current seasonal influenza vaccines have varying effectiveness [5], antiviral drugs are an essential complement for influenza prevention, treatment and pandemic preparedness [6]. As of today, three drug classes are approved. The adamantane derivatives (amantadine and rimantadine) block the M2 ion channel of influenza A virus but are no longer recommended for clinical use, due to widespread viral resistance against these inhibitors [7]. The neuraminidase inhibitors oseltamivir, zanamivir, laninamivir and peramivir prevent the release of progeny virions from infected cells. In many countries, oseltamivir is the standard-of-care for influenza A and B, however resistance against this drug needs to be closely monitored [8]. In recent years, inhibitors of the viral polymerase complex have received major attention, with favipiravir and baloxavir marboxil already approved in several countries [9,10,11,12]. In Russia and China, the broad-spectrum antiviral drug arbidol (also known as umifenovir) has been available since many years [13, 14]. This molecule is the only approved inhibitor of influenza virus entry. Besides other potential mechanisms [15], arbidol acts by preventing the conformational change of the HA trimer at low pH [16,17,18]. After the virus has entered by endocytosis, HA refolding is required to release the fusion peptide and trigger fusion of the viral and endosomal membranes [19]. The literature contains numerous small molecule inhibitors of HA refolding [reviewed in: [20, 21]], however their subtype-dependent or group-specific [22] anti-influenza A virus activity form a main obstacle for preclinical development.
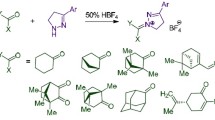
For over ten years now, research efforts in our laboratory have been focused on the synthesis and antiviral evaluation of compounds containing a spirocyclic ring system. In 2010, we identified a series of influenza virus fusion inhibitors with spirothiazolidinone (1-thia-4-azaspiro[4.5]decane) scaffold and strong cell culture activity against influenza A/H3N2 virus. Mechanistic studies established that these spirothiazolidinone compounds prevent the conformational change of H3 HA at low pH [23, 24]. These inhibitors share a common framework, consisting of an aromatic ring linked to a spirothiazolidinone system via an amide bridge. The lead compound A, identified in 2010, bears an imidazo[2,1-b]thiazole scaffold as the aromatic part (Fig. 1) [23]. Subsequent structure–activity relationship (SAR) studies demonstrated that the anti-A/H3N2 activity was maintained when the aromatic part was replaced by a substituted phenyl group (Fig. 1), i.e. o-hydroxyphenyl (B) [23], 5-chloro-2-hydroxyphenyl (C) [25], 5-chloro-2-methoxyphenyl (D) [26], 4-chlorophenoxymethyl (E) [27] or 1-adamantyl (F) [24], 2-methylfuran-3-yl (G) [28], 5-chloro-3-methyl-indole-2-yl (H) [18]. To date, all these spirothiazolidinone compounds exhibit narrow activity against influenza A/H3 HA, with no inhibition of A/H1, A/H5 and A/H7 nor of influenza B HA [23].
Based on our previous biological results, we designed a new series of benzamide-derived spiro compounds with a methoxy substituent at ortho position and an electron-withdrawing sulfonamide group at para position. The sulfonamide group is important in medicinal chemistry, since a wide variety of drugs have the benzene sulfonamide nucleus. Antiviral activity is also observed in a wide range of compounds with a sulfonamide fragment in combination with aromatic or heteroaromatic rings [29, 30]. Therefore, we decided to examine the influence of introducing the sulfonamide moiety. We here report the chemical synthesis, structural characterization and antiviral evaluation of this series of 2-methoxy-N-(2-methyl-3-oxo-1-thia-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamides (3a–j) against influenza A/H1N1, A/H3N2 and B viruses, as well as herpes simplex virus type 1 (HSV-1), respiratory syncytial virus (RSV) and yellow fever virus (YFV).
Results and discussion
Chemistry
The synthetic pathway for the preparation of new spirothiazolidinones (3a–j) is demonstrated in Scheme 1. Three experimental approaches have been described in the literature for spirothiazolidinone cyclization: traditional two-step method [31, 32], greener one-pot method [23, 27, 28] and microwave-assisted green one-pot synthesis [24, 26]. In this study, new spirocyclic compounds were obtained by one-pot reaction. Thus, the key intermediate 4-(aminosulfonyl)-2-methoxybenzohydrazide (2) was reacted with an appropriate cyclic ketone and 2-mercaptopropionic acid in one-pot, using a Dean Stark water separator. The novel compounds (3a–j) were characterized by combustion analysis and IR, 1H NMR and 13C NMR (APT) spectral studies.
The detailed spectral data of compounds 3a–j are shown in the experimental section. The solid phase (KBr) IR spectra of 3a–j showed common characteristic absorption bands at 3336–3211 cm−1 (N–H stretching bands), 1699–1685 cm−1 (lactam C=O bands) and 1674–1639 cm−1 (amide C=O bands), which provided evidence for the cycloaddition reaction. The 1H NMR spectra of the synthesized compounds showed characteristic broad singlets of benzamide NH group at δ 10.54–10.26 ppm. The S-CH protons of the newly formed thiazolidinone residue resonated as quartets at δ 4.04–3.90 ppm, confirming the structure of the desired compounds. The remaining proton signals of spiroalkane system were detected at δ 3.48–0.68 ppm region, together with the alkyl substituents. The resonances of 2-OCH3 and 4-SO2NH2 groups at the phenyl subunit were observed as singlets in the δ 3.98–3.92 ppm and δ 7.39–7.34 ppm, respectively. Peaks associated with the aromatic ring were observed in the expected regions (δ 8.06–7.32 ppm) and the splitting patterns were in accordance with the 1,2,4-trisubstituted aromatic ring system. APT spectra of 3a–j showed two downfield signals at about δ 170.5–164.6 ppm due to the carbonyl carbon absorptions. Observation of upfield resonances assigned to the aliphatic CH/CH2 carbons and the typical spirodecan C5 resonances (δ 71.6–69.5 ppm) substantiated the formation of the expected spirothiazolidinones.
Antiviral activity
The anti-influenza virus activity of the ten new spirocyclic compounds was determined in Madin-Darby canine kidney (MDCK) cells, using two strains of influenza A virus [A/Virginia/ATCC3/2009 (A/H1N1) and A/HK/7/87 (A/H3N2)] and one strain of influenza B virus [B/Ned/537/05]. In addition, the compounds were evaluated against HSV-1 and RSV in HEL299 cells and against YFV in Huh7 cells. The 50% effective concentration (EC50) was defined as the compound concentration producing 50% inhibition of virus-induced cytopathic effect (CPE), as assessed by microscopic scoring and MTS cell viability assay. In parallel, compound cytotoxicity was determined in mock-infected cultures, and expressed as minimal cytotoxic concentration (MCC, based on microscopy) and 50% cytotoxic concentration (CC50, by MTS assay) (Table 1).
Since our previous studies indicate that the anti-A/H3N2 activity of the spirothiazolidinone compounds is preserved for several variations of the aromatic part (see above), it is quite surprising that neither of the new compounds had noticeable activity against A/H3N2 virus (Table 1). They all carry a methyl group at position 2 in the spiro ring, which we proved to be a crucial substituent [18, 23]. Also, the new compounds 3b (8-methyl) and 3c (8-ethyl) are the direct analogues of the highly potent and published compounds 4c and 4d [23] and 5e and 5f [18], the only difference being the structure of the aromatic part. On the other hand, two of the new compounds displayed weak activity against A/H1N1 virus, with compound 3d (8-propyl) and 3e (8-tert-butyl) having EC50 values in the range of 35–45 µM and no cytotoxicity at 100 μM, the highest concentration tested. At this concentration, neither of the compounds had an effect on the replication of HSV-1, RSV or YFV (data not shown).
Conclusion
A series of novel spirothiazolidinone compounds carrying 2-methoxy-4-sulfamoylbenzamide moiety (3a–j) have been synthesized, characterized, and evaluated as replication inhibitors of influenza virus. Two compounds (3d and 3e) displayed weak activity against influenza A/H1N1 virus. This is unexpected since, so far, our spirothiazolidinone class of fusion inhibitors was active against A/H3N2 but not A/H1N1 virus. This includes the previously synthesized o-hydroxy and o-methoxy substituted phenyl derivatives (B, C and D in Fig. 1). Most likely, the electron-withdrawing sulfonamide group on the para position of the benzene ring is responsible for the change in anti-influenza virus activity profile. On the other hand, our new data indicate that the inhibitory activity of the spirothiazolidinone class is amenable towards specific HA subtypes, with even subtle differences having the ability to change the binding properties in the HA binding pocket [18]. Hence, we have embarked on further structural optimization of the aromatic part, to conduct mechanistic antiviral experiments and hopefully increase the anti-A/H1N1 activity.
Experimental section
Materials
Chemicals were obtained from Sigma Aldrich. Reaction progress was monitored by thin layer chromatography (TLC) using silica gel plates and chloroform:methanol (9:1) as the eluent. Melting points (mp) were determined on a Buchi B-540 capillary melting point apparatus in open capillaries and uncorrected. IR spectra were recorded in KBr discs on a Shimadzu IR Affinity-1 FTIR. 1H NMR (DMSO-d6) spectra were run on a VarianMERCURY400 MHZ and 13C NMR (APT) (DMSO-d6) spectra were run Bruker 500 MHz spectrophotometers. Microanalyses were performed on a Thermo Finnigan Flash EA 1112 elemental analyzer. (Sp: spirothiazolidinone, Ar: aromatic ring).
Chemical synthesis
General procedure for the synthesis of compound 4-(aminosulfonyl)-2-methoxybenzohydrazide (2)
To the solution of 0.05 mol of methyl 2-methoxy-4-sulfamoylbenzoate (1) in 10 mL of ethanol was added 0.1 mol of 99% hydrazine hydrate. The mixture was refluxed for 2 h. The reaction mixture was then cooled, diluted with water and allowed to stand overnight and used as a crude product. Rf (2) = 0.22.
General procedure for the synthesis of compounds 3a-j
A mixture of 2 (0.005 mol) and appropriate ketone (0.01 mol) in 30 mL of dried toluene were refluxed for 1 h, using a Dean Stark water separator. After 1 h, 2-sulfanylpropanoic acid (0.01 mol) was added and the mixture was refluxed during 6–8 h. Excess toluene was evaporated in vacuo. The resulting residue was treated with saturated NaHCO3 solution until CO2 evolution ceased and was allowed to stand overnight or in some cases refrigerated until solidification. The precipitate was filtered and purified by recrystallization from ethanol.
2-Methoxy-N-(2,7-dimethyl-3-oxo-1-thia-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamide (3a)
White powder (97%); Rf (3a) = 0.57; m.p: 261–264 °C; IR (KBr): υmax 3294, 3211 (N–H), 1693 (C=O), 1662 (NHC=O), 1342, 1168 (S=O). 1H NMR (DMSO-d6/400 MHz): δ 10.26 (1H, s, NH), 7.99, 8.00 (1H, 2d, J = 2.4 Hz, Ar-H3), 7.91 (1H, dd, J = 8.8, 2.4 Hz, Ar-H5), 7.34 (2H, s, SO2NH2), 7.32 (1H, d, J = 8.9 Hz, Ar-H6), 3.93 (3H, s, OCH3), 3.91 (1H, q, J = 7.0 Hz, Sp-S-CH), 1.91–1.46 (8H, m, Sp-CH/CH2), 1.43 (3H, d, J = 6.8 Hz, Sp-2-CH3), 0.89 (3H, d, J = 6.0 Hz, Sp-7-CH3), 0.80–0.68 (1H, m, Sp-CH/CH2). 13C NMR (DMSO-d6/125 MHz): 170.4 (Sp-CO), 164.7 (NHCO), 159.4 (Ar-C2), 136.6 (Ar-C4), 130.6, 130.5, 128.3, 128.2 (Ar-C5,C6), 123.2, 123.1 (Ar-C1), 112.8 (Ar-C3), 71.4 (Ar-C5), 57.1, 57.0 (OCH3), 46.8, 46.5 (Sp-CH2), 37.9, 36.9, 33.4, (Sp-CH2), 37.3, 37.2 (Sp-C2), 30.3, 29.8 (Sp-C7), 23.20 (Sp-CH2), 22.7, 22.5 (Sp-7-CH3), 20.2, 19.9 (Sp-2-CH3). Anal. calcd. for C18H25N3O5S2 (427.53) C: 50.57, H: 5.89, N: 9.83. Found C: 50.52, H:6.20, N: 9.75.
2-Methoxy-N-(2,8-dimethyl-3-oxo-1-thia-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamide (3b)
White powder (56%); Rf (3b) = 0.64; m.p: 258–261 °C; IR (KBr): υmax 3321, 3219 (N–H), 1691 (C=O), 1641 (NHC=O), 1323, 1159 (S=O). 1H NMR (DMSO-d6/400 MHz): δ 10.32 (1H, s, NH), 8.03 (1H, d, J = 2.4 Hz, Ar-H3), 7.95 (1H, dd, J = 8.8, 2.4 Hz, Ar-H5), 7.39 (2H, s, SO2NH2), 7.35 (1H, d, J = 8.9 Hz, Ar-H6), 3.96 (3H, s, OCH3), 3.94 (1H, q, J = 7.0 Hz, Sp-S-CH), 2.08–1.70 (6H, m, Sp-CH/CH2), 1.46 (3H, d, J = 7.0 Hz, Sp-2-CH3), 1.36–1.07 (3H, m, Sp-CH/CH2), 0.90 (3H, d, J = 6.2 Hz, Sp-8-CH3). 13C NMR (DMSO-d6/125 MHz): 170.5 (Sp-CO), 164.7 (NHCO), 159.5 (Ar-C2), 136.6 (Ar-C4), 130.5, 128.2 (Ar-C5, C6), 123.1 (Ar-C1), 112.8 (Ar-C3), 71.3 (Ar-C5), 57.0 (OCH3), 38.1 (Sp-CH2), 37.2 (Sp-C2), 32.1, 31.7 (Sp-CH2), 31.2 (Sp-C8), 22.3 (Sp-8-CH3), 20.1 (Sp-2-CH3). Anal. calcd. for C18H25N3O5S2 (427.53) C: 50.57, H: 5.89, N: 9.83. Found C: 50.57, H: 5.86, N: 9.88.
2-Methoxy-N-(8-ethyl-2-methyl-3-oxo-1-thia-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamide (3c)
White powder (98%); Rf (3c) = 0.40; m.p: 270–272 °C; IR (KBr): υmax 3317, 3219 (N–H), 1689 (C=O), 1645 (NHC=O), 1321, 1159 (S=O). 1H NMR (DMSO-d6/400 MHz): δ 10.27 (1H, s, NH), 8.00 (1H, d, J = 2.4 Hz, Ar-H3), 7.91 (1H, dd, J = 8.8, 2.4 Hz, Ar-H5), 7.34 (2H, s, SO2NH2), 7.32 (1H, d, J = 8.8 Hz, Ar-H6), 3.93 (3H, s, OCH3), 3.90 (1H, q, J = 7.0 Hz, Sp-S-CH), 2.03–1.69 (6H, m, Sp-CH/CH2), 1.42 (3H, d, J = 6.8 Hz, Sp-2-CH3), 1.27–1.00 (5H, m, Sp-CH/CH2, 8-CH2CH3), 0.84 (3H, t, J = 7.4 Hz, Sp-8-CH2CH3). 13C NMR (DMSO-d6/125 MHz): 170.4 (Sp-CO), 164.7 (NHCO), 159.5 (Ar-C2), 136.6 (Ar-C4), 130.5, 128.2 (Ar-C5, C6), 123.1 (Ar-C1), 112.8 (Ar-C3), 71.6 (Ar-C5), 57.0 (OCH3), 38.1, 29.6, 29.2 (Sp-CH2, Sp-8-CH2CH3), 37.7, 37.2 (Sp-C2, C8), 20.1 (Sp-2-CH3), 11.8 (Sp-8-CH2CH3). Anal. calcd. for C19H27N3O5S2 (441.56) C: 51.68, H: 6.16, N: 9.52. Found C: 51.29, H: 6.26, N: 9.46.
2-Methoxy-N-(2-methyl-3-oxo-8-propyl-1-thia-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamide (3d)
White powder (88%); Rf (3d) = 0.44; m.p: 274–276 °C; IR (KBr): υmax 3315, 3221 (N–H), 1689 (C=O), 1645 (NHC=O), 1319, 1159 (S=O). 1H NMR (DMSO-d6/400 MHz): δ 10.30 (1H, s, NH), 8.00 (1H, d, J = 2.4 Hz, Ar-H3), 7.91 (1H, dd, J = 8.7, 2.5 Hz, Ar-H5), 7.36 (2H, s, SO2NH2), 7.32 (1H, d, J = 8.8 Hz, Ar-H6), 3.92 (3H, s, OCH3), 3.90 (1H, q, J = 7.0 Hz, Sp-S-CH), 2.03–1.68 (6H, m, Sp- CH/CH2), 1.42 (3H, d, J = 7.0 Hz, Sp-2-CH3), 1.34–0.98 (7H, m, Sp-CH/CH2, 8-CH2CH2CH3), 0.84 (3H, t, J = 7.2 Hz, Sp-8-CH2CH2CH3). 13C NMR (DMSO-d6/125 MHz): 170.5 (Sp-CO), 164.7 (NHCO), 159.5 (Ar-C2), 136.6 (Ar-C4), 130.5, 128.3 (Ar-C5, C6), 123.1 (Ar-C1), 112.8 (Ar-C3), 71.6 (Sp-C5), 56.9 (OCH3), 38.8, 38.1, 30.1, 29.6 (Sp-CH2, Sp-8-CH2CH2CH3), 37.2 (Sp-C2), 35.7 (Sp-C8), 20.1 (Sp-2-CH3), 19.9 (Sp-8-CH2CH2CH3), 14.6 (Sp-8-CH2CH2CH3). Anal. calcd. for C20H29N3O5S2 (455.59) C: 52.73, H: 6.42, N: 9.22. Found C: 52.58, H: 6.65, N: 9.30.
2-Methoxy-N-(2-methyl-3-oxo-8-tert-butyl-1-thia-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamide (3e)
White powder (68%); Rf (3e) = 0.46; m.p: 301–304 °C; IR (KBr): υmax 3336, 3302, 3219 (N–H), 1691 (C=O), 1651 (NHC=O), 1323, 1159 (S=O). 1H NMR (DMSO-d6/400 MHz): δ 10.26 (1H, s, NH), 8.02 (1H, d, J = 2.4 Hz, Ar-H3), 7.92 (1H, dd, J = 8.7, 2.5 Hz, Ar-H5), 7.34 (2H, s, SO2NH2), 7.32 (1H, d, J = 8.9 Hz, Ar-H6), 3.93 (3H, s, OCH3), 3.90 (1H, q, J = 7.0 Hz, Sp-S-CH), 2.01–1.71 (6H, m, Sp-CH/CH2), 1.43 (3H, d, J = 7.0 Hz, Sp-2-CH3), 1.31–1.11 (2H, m, Sp-CH/CH2), 0.96–0.87 (1H, m, Sp-CH/CH2), 0.83 (9H, s, Sp-8-C(CH3)3). 13C NMR (DMSO-d6/125 MHz): 170.5 (Sp-CO), 164.7 (NHCO), 159.5 (Ar-C2), 136.6 (Ar-C4), 130.6, 128.3 (Ar-C5, C6), 123.0 (Ar-C1), 112.8 (Ar-C3), 71.5 (Sp-C5), 57.0 (OCH3), 46.4 (Sp-C8), 38.4, 37.6, 24.5, 24.0 (Sp-CH2), 37.2 (Sp-C2), 32.4 (Sp-8-C(CH3)3), 27.7 (Sp-8-C(CH3)3), 20.1 (Sp-2-CH3). Anal. calcd. for C21H31N3O5S2 (469.61) C: 53.71, H: 6.65, N: 8.95. Found C: 53.73, H: 6.74, N: 9.11.
2-Methoxy-N-(2-methyl-3-oxo-8-tert-pentyl-1-thia-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamide (3f)
White powder (72%); Rf (3f) = 0.67; m.p: 290–295 °C; IR (KBr): υmax 3321, 3223 (N–H), 1691 (C=O), 1651 (NHC=O), 1328, 1159 (S=O). 1H NMR (DMSO-d6/400 MHz): δ 10.28 (1H, s, NH), 8.01 (1H, d, J = 2.4 Hz, Ar-H3), 7.92 (1H, dd, J = 8.8, 2.5 Hz, Ar-H5), 7.36 (2H, s, SO2NH2), 7.32 (1H, d, J = 8.8 Hz, Ar-H6), 3.92 (3H, s, OCH3), 3.90 (1H, q, J = 7.0 Hz, Sp-S-CH), 2.02–1.64 (6H, m, Sp-CH/CH2), 1.42 (3H, d, J = 7.0 Hz, Sp-2-CH3), 1.33–1.11 (2H, m, Sp-CH/CH2), 1.22 (2H, q, J = 7.6 Hz, Sp-8-C(CH3)2CH2CH3), 1.04–0.92 (1H, m, Sp-CH/CH2), 0.77 (6H, s, Sp-8-C(CH3)2CH2CH3), 0.70 (3H, t, J = 7.6 Hz, Sp-8-C(CH3)2CH2CH3). 13C NMR (DMSO-d6/125 MHz): 170.5 (Sp-CO), 164.7 (NHCO), 159.5 (Ar-C2), 136.7 (Ar-C4), 130.5, 128.3 (Ar-C5, C6), 123.0 (Ar-C1), 112.8 (Ar-C3), 71.5 (Sp-C5), 43.7 (Sp-C8), 38.5, 37.6 (Sp-CH2), 37.2 (Sp-C2), 34.6, 32.6 (Sp-8-C(CH3)2CH2CH3), 24.53 (Sp-8-C(CH3)2CH2CH3), 24.0, 23.6 (Sp-CH2), 20.1 (Sp-2-CH3), 8.4 (Sp-8-C(CH3)2CH2CH3). Anal. calcd. for C22H33N3O5S2 (483.64) C: 54.63, H: 6.88, N: 8.69. Found C: 54.94, H: 7.29, N: 8.70.
2-Methoxy-N-(2-methyl-3-oxo-1-thia-8-trifluoromethyl-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamide (3 g)
White powder (78%); Rf (3 g) = 0.45; m.p: 210–214 °C; IR (KBr): υmax 3311, 3221 (N–H), 1689 (C=O), 1647 (NHC=O), 1390, 1159 (S=O). 1H NMR (DMSO-d6/400 MHz): δ 10.31 (1H, s, NH), 8.06 (1H, d, J = 2.4 Hz, Ar-H3), 7.93 (1H, dd, J = 8.8, 2.5 Hz, Ar-H5), 7.36 (2H, s, SO2NH2), 7.33 (1H, d, J = 8.9 Hz, Ar-H6), 3.97 (1H, q, J = 7.0 Hz, Sp-S-CH), 3.95 (3H, s, OCH3), 2.38–2.18 (1H, m, Sp-CH/CH2), 2.11–1.70 (6H, m, Sp-CH/CH2), 1.62–1.36 (2H, m, Sp-CH/CH2), 1.43 (3H, d, J = 7.0 Hz, Sp-2-CH3). 13C NMR (DMSO-d6/125 MHz): 170.4 (Sp-CO), 164.7 (NHCO), 159.6 (Ar-C2), 136.6 (Ar-C4), 130.8, 128.5 (Ar-C5, C6), 128.2 (q, J = 277.0 Hz, Sp-8-CF3), 122.6 (Ar-C1), 112.9 (Ar-C3), 70.3 (Sp-C5), 57.1 (OCH3), 39.0 (d, J = 26 Hz, Sp-C8), 37.3 (Sp-C2), 36.3, 35.5, 22.4, 21.9 (Sp-CH2), 19.9 (Sp-2-CH3). Anal. calcd. for C18H22F3N3O5S2 (481.50) C: 44.90, H: 4.61, N: 8.73. Found C: 44.69, H: 4.60, N: 8.82.
2-Methoxy-N-(2-methyl-3-oxo-8-phenyl-1-thia-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamide (3 h)
White powder (79%); Rf (3 h) = 0.56; m.p: 290–292 °C; IR (KBr): υmax 3304, 3217 (N–H), 1685 (C=O), 1639 (NHC=O), 1319, 1159 (S=O). 1H NMR (DMSO-d6/400 MHz): δ 10.35 (1H, s, NH), 8.06 (1H, d, J = 2.4 Hz, Ar-H3), 7.94 (1H, dd, J = 8.8, 2.5 Hz, Ar-H5), 7.36 (2H, s, SO2NH2), 7.35 (1H, d, J = 8.9 Hz, Ar-H6), 7.31–7.14 (5H, m, Sp-8-C6H5), 3.98 (3H, s, OCH3), 3.96 (1H, q, J = 7.0 Hz, Sp-S-CH), 2.50–2.42 (m, DMSO-d6 and Sp-C8-H), 2.23–1.84 (6H, m, Sp-CH/CH2), 1.78–1.55 (2H, m, Sp-CH/CH2), 1.46 (3H, d, J = 7.0 Hz, Sp-2-CH3). 13C NMR (DMSO-d6/125 MHz): 170.5 (Sp-CO), 164.8 (NHCO), 159.6 (Ar-C2), 146.2 (Sp-8-C6H5(C1)), 136.7 (Ar-C4), 130.6, 128.4 (Ar-C5, C6), 128.4, 127.2, 126.6 (Sp-8-C6H5(C2-6)), 122.9 (Ar-C1), 112.9 (Ar-C3), 71.0 (Sp-C5), 57.1 (OCH3), 42.1 (Sp-C8), 38.4, 37.5 (Sp-CH2), 37.3 (Sp-C2), 31.3, 30.7 (Sp-CH2), 20.1 (Sp-2-CH3). Anal. calcd. for C23H27N3O5S2 (489.60) C: 56.42, H: 5.56, N: 8.58. Found C: 56.34, H: 5.70, N: 8.59.
2-Methoxy-N-(8-cyano-2-methyl-3-oxo-8-phenyl-1-thia-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamide (3i)
White powder (75%); Rf (3i) = 0.36; m.p: 265–270 °C; IR (KBr): υmax 3261 (N–H), 1699 (C=O), 1672 (NHC=O), 1334, 1165 (S=O). 1H NMR (DMSO-d6/400 MHz): δ 10.54 (1H, s, NH), 7.98 (1H, d, J = 2.5 Hz, Ar-H3), 7.93 (1H, dd, J = 8.7, 2.5 Hz, Ar-H5), 7.53 (2H, d, J = 7.6 Hz, 8-C6H5(H2,H6), 7.45 (2H, t, J = 7.6 Hz, 8-C6H5(H3,H5), 7.40–7.33 (4H, m, Sp-8-C6H5(H4), SO2NH2 and Ar-H6), 4.04 (1H, q, J = 7.0 Hz, Sp-S-CH), 3.97 (3H, s, OCH3), 2.60–2.33 (m, DMSO-d6 and Sp-CH2), 2.14–1.93 (4H, m, Sp-CH2), 1.47 (3H, d, J = 7.0 Hz, Sp-2-CH3). 13C NMR (DMSO-d6/125 MHz): 170.4 (Sp-CO), 165.1 (NHCO), 159.4 (Ar-C2), 140.2 (Sp-8-C6H5(C1)), 36.6 (Ar-C4), 130.5, 128.0 (Ar-C5,C6), 129.4, 128.7, 126.2 (Sp-8-C6H5(C2-6)), 122.2 (Ar -C1), 122.2 (Sp-4-CN), 112.7 (Ar-C3), 69.7 (Sp-C5), 57.0 (OCH3), 42.3 (Sp-C8), 37.4 (Sp-C2), 35.6, 34.9, 34.1, 33.5 (Sp-CH2), 20.0 (Sp-2-CH3). Anal. calcd. for C24H26N4O5S2.H2O (532.57) C: 54.12, H: 5.30, N: 10.89. Found C: 53.99, H: 5.11, N: 10.52.
2-Methoxy-N-(8-acetamido-2-methyl-3-oxo-1-thia-4-azaspiro[4.5]decan-4-yl)-4-sulfamoylbenzamide (3j)
White powder (90%); Rf (3j) = 0.60; m.p: 220–223 °C; IR (KBr): υmax 3269, 3228 (N–H), 1699 (C=O), 1674, 1622 (NHC=O), 1340, 1165 (S=O). 1H NMR (DMSO-d6/400 MHz): δ 10.34 (1H, s, NH), 8.00 (1H, d, J = 2.5 Hz, Ar-H3), 7.92 (1H, dd, J = 8.8, 2.5 Hz, Ar-H5), 7.83 (1H, d, J = 7.7 Hz, NHCOCH3), 7.36 (2H, s, SO2NH2), 7.33 (1H, d, J = 8.9 Hz, Ar-H6), 3.96 (1H, q, J = 7.0 Hz, Sp-S-CH), 3.94 (3H, s, OCH3), 3.48–3.36 (1H, m, Sp-C8-H), 2.13–1.76 (6H, m, Sp-CH2), 1.75 (3H, s, NHCOCH3), 1.51–1.29 (2H, m, sp-CH2), 1.43 (3H, d, J = 7.0 Hz, Sp-2-CH3). 13C NMR (DMSO-d6/125 MHz): 170.5 (Sp-CO), 164.9, 164.6 (NHCO, NHCOCH3), 159.5 (Ar-C2), 136.9, 136.7, 136.0 (Ar-C4), 130.0, 128.9, 127.2 (Ar-C5,C6), 123.1 (Ar-C1), 112.9 (Ar-C3), 69.5 (Sp-C5), 57.3 (OCH3), 37.2 (Sp-C2), 35.4 (Sp-C8), 29.4, 29.1 (Sp-CH2), 21.6 (Sp-8-NHCOCH3), 19.9 (Sp-2-CH3). Anal. calcd. for C16H22N4O5S. 1/2H2O (391.43) C: 49.10, H: 6.13, N: 14.32. Found C: 49.62, H: 5.89, N: 14.50.
Antiviral procedures
The influenza virus cytopathic effect (CPE) reduction assay was reported in full detail elsewhere [33]. Briefly, Madin-Darby canine kidney (MDCK) cells were seeded in 96-well plates at 7,500 cells per well, using Ultra-MDCK medium (from Lonza) supplemented with 2 µg per ml of trypsin. On the next day, they were infected with 100 CCID50 (50% cell culture infective dose) per well of influenza A/H1N1 (A/Virginia/ATCC3/2009) or A/H3N2 (A/HK/7/87) virus (both from ATCC), or influenza B virus (B/Ned/537/05; kind gift from R. Fouchier). At the same time, the compounds were added at serial dilutions. Mock-infected plates prepared in parallel received the compounds but no virus. After four days incubation at 35 °C, the virus-induced CPE was scored by microscopy, after which the colorimetric MTS assay (CellTiter 96® AQueous One Solution Cell Proliferation Assay from Promega) was conducted. The same two methods were applied to the mock-infected plate, to determine compound cytotoxicity. Antiviral activity was defined as the 50% effective concentration (EC50), whereas cytotoxicity was expressed as MCC (minimal cytotoxic concentration, based on microscopy) or CC50 (50% cytotoxic concentration, assessed by the MTS assay) [see reference (28) for calculation methods].
Analogous CPE reduction assays were used for HSV-1 (strain KOS) and RSV (strain Long), both assessed in HEL299 human embryonic lung fibroblast cells, and for YFV (strain 17D), assessed in Huh-7 human liver carcinoma cells. After infection and compound addition, the cells were incubated for 3–6 days at 37 °C, until full-blown CPE was visible. The inhibitory effect on virus-induced CPE and compound cytotoxicity was determined by microscopy and MTS assay, and the data were analyzed as above.
References
Uyeki TM, Hui DS, Zambon M, Wentworth DE, Monto AS (2022) Influenza. Lancet 400:693–706. https://doi.org/10.1016/S0140-6736(22)00982-5
Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, Doherty PC, Palese P, Shaw ML, Treanor J, Webster RG, Garcia-Sastre A (2018) Influenza. Nat Rev Dis Primers 4:3. https://doi.org/10.1038/s41572-018-0002-y
Petrova VN, Russell CA (2018) The evolution of seasonal influenza viruses. Nat Rev Microbiol 16:47–60. https://doi.org/10.1038/nrmicro.2017.118
de Vries E, Du W, Guo H, de Haan CAM (2020) Influenza A virus hemagglutinin-neuraminidase-receptor balance: preserving virus motility. Trends Microbiol 28:57–67. https://doi.org/10.1016/j.tim.2019.08.010
Treanor JJ (2016) Clinical practice, influenza vaccination. N Engl J Med 375:1261–1268. https://doi.org/10.1056/NEJMcp1512870
Beigel JH, Hayden FG (2021) Influenza therapeutics in clinical practice-challenges and recent advances. Cold Spring Harb Perspect Med 11:a038463. https://doi.org/10.1101/cshperspect.a038463
Uyeki TM, Bernstein HH, Bradley JS, Englund JA, File TM, Fry AM, Gravenstein S, Hayden FG, Harper SA, Hirshon JM, Ison MG, Johnston BL, Knight SL, McGeer A, Riley LE, Wolfe CR, Alexander PE, Pavia AT (2019) Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis 68:895–902. https://doi.org/10.1093/cid/ciy866
Govorkova EA, Takashita E, Daniels RS, Fujisaki S, Presser LD, Patel MC, Huang W, Lackenby A, Nguyen HT, Pereyaslov D, Rattigan A, Brown SK, Samaan M, Subbarao K, Wong S, Wang D, Webby RJ, Yen HL, Zhang W, Meijer A, Gubareva LV (2022) Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2018–2020. Antiviral Res 200:105281. https://doi.org/10.1016/j.antiviral.2022.105281
Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL (2013) Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100:446–454. https://doi.org/10.1016/j.antiviral.2013.09.015
Hayden FG, Lenk RP, Stonis L, Oldham-Creamer C, Kang LL, Epstein C (2022) Favipiravir treatment of uncomplicated influenza in adults: results of two Phase 3, randomized, double-blind, placebo-controlled trials. J Infect Dis 226:1790–1799. https://doi.org/10.1093/infdis/jiac135
Uehara T, Hayden FG, Kawaguchi K, Omoto S, Hurt AC, De Jong MD, Hirotsu N, Sugaya N, Lee N, Baba K, Shishido T, Tsuchiya K, Portsmouth S, Kida H (2020) Treatment-emergent influenza variant viruses with reduced baloxavir susceptibility: impact on clinical and virologic outcomes in uncomplicated influenza. J Infect Dis 221:346–355. https://doi.org/10.1093/infdis/jiz244
Stevaert A, Groaz E, Naesens L (2022) Nucleoside analogs for management of respiratory virus infections: mechanism of action and clinical efficacy. Curr Opin Virol 57:101279
Ruzhentsova TA, Oseshnyuk RA, Soluyanova TN, Dmitrikova EP, Mustafaev DM, Pokrovskiy KA, Markova TN, Rusanova MG, Kostina NE, Agafina AS, Brook YF, Bronov OY, Shults EI, Filon OV (2021) Phase 3 trial of coronavir (favipiravir) in patients with mild to moderate COVID-19. Am J Transl Res 13:12575–12587
Leneva IA, Falynskova IN, Makhmudova NR, Poromov AA, Yatsyshina SB, Maleev VV (2019) Umifenovir susceptibility monitoring and characterization of influenza viruses isolated during ARBITR clinical study. J Med Virol 91:588–597. https://doi.org/10.1002/jmv.25358
Teissier E, Zandomeneghi G, Loquet A, Lavillette D, Lavergne JP, Montserret R, Cosset FL, Bockmann A, Meier BH, Penin F, Pecheur EI (2011) Mechanism of inhibition of enveloped virus membrane fusion by the antiviral drug arbidol. PLoS ONE 6:e15874. https://doi.org/10.1371/journal.pone.0015874
Kadam RU, Wilson IA (2017) Structural basis of influenza virus fusion inhibition by the antiviral drug arbidol. Proc Natl Acad Sci USA 114:206–214. https://doi.org/10.1073/pnas.1617020114
Boriskin YS, Leneva IA, Pecheur EI, Polyak SJ (2008) Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem 15:997–1005. https://doi.org/10.2174/092986708784049658
Cihan-Ustundag G, Zopun M, Vanderlinden E, Ozkirimli E, Persoons L, Capan G, Naesens L (2020) Superior inhibition of influenza virus hemagglutinin-mediated fusion by indole-substituted spirothiazolidinones. Bioorg Med Chem 28:115130. https://doi.org/10.1016/j.bmc.2019.115130
Gamblin SJ, Vachieri SG, Xiong X, Zhang J, Martin SR, Skehel JJ (2020) Hemagglutinin structure and activities. Cold Spring Harb Perspect Med 11:a038638. https://doi.org/10.1101/cshperspect.a038638
Vanderlinden E, Naesens L (2014) Emerging antiviral strategies to interfere with influenza virus entry. Med Res Rev 34:301–339. https://doi.org/10.1002/med.21289
Chen Z, Cui Q, Caffrey M, Rong L, Du R (2021) Small molecule inhibitors of influenza virus entry. Pharmaceuticals (Basel) 14:587. https://doi.org/10.3390/ph14060587
van Dongen MJP, Kadam RU, Juraszek J, Lawson E, Brandenburg B, Schmitz F, Schepens WBG, Stoops B, van Diepen HA, Jongeneelen M, Tang C, Vermond J, van Eijgen-Obregoso RA, Blokland S, Garg D, Yu W, Goutier W, Lanckacker E, Klap JM, Peeters DCG, Wu J, Buyck C, Jonckers THM, Roymans D, Roevens P, Vogels R, Koudstaal W, Friesen RHE, Raboisson P, Dhanak D, Goudsmit J, Wilson IA (2019) A small-molecule fusion inhibitor of influenza virus is orally active in mice. Science 363:1056. https://doi.org/10.1126/science.aar6221
Vanderlinden E, Göktas F, Cesur Z, Froeyen M, Reed ML, Russell CJ, Cesur N, Naesens L (2010) Novel inhibitors of influenza virus fusion: structure-activity relationship and interaction with the viral hemagglutinin. J Virol 84:4277–4288. https://doi.org/10.1128/JVI.02325-09
Goktas F, Vanderlinden E, Naesens L, Cesur N, Cesur Z (2012) Microwave assisted synthesis and anti-influenza virus activity of 1-adamantyl substituted N-(1-thia-4-azaspiro[4.5]decan-4-yl)carboxamide derivatives. Bioorg Med Chem 20:7155–7159. https://doi.org/10.1016/j.bmc.2012.09.064
Goktas F, Vanderlinden E, Naesens L, Cesur Z, Cesur N, Tas P (2015) Synthesis and structure-activity relationship of N-(3-oxo-1-thia-4-azaspiro[4.5] decan-4-yl)carboxamide inhibitors of influenza virus hemagglutinin mediated fusion. Phosphorus Sulfur Silicon Relat Elem 190:1075–1087. https://doi.org/10.1080/10426507.2014.965819
Goktas F, Ozbil M, Cesur N, Vanderlinden E, Naesens L, Cesur Z (2019) Novel N-(1-thia-4-azaspiro[4.5]decan-4-yl)carboxamide derivatives as potent and selective influenza virus fusion inhibitors. Arch Pharm (Weinheim) 352:e1900028. https://doi.org/10.1002/ardp.201900028
Apaydın ÇB, Van Loy B, Stevaert A, Naesens L (2020) New spirothiazolidinone derivatives: synthesis and antiviral evaluation. Phosphorus Sulfur Silicon Relat Elem 196:294–299. https://doi.org/10.1080/10426507.2020.1828886
Apaydın ÇB, Tansuyu M, Cesur Z, Naesens L, Göktaş F (2021) Design, synthesis and anti-influenza virus activity of furan-substituted spirothiazolidinones. Bioorg Chem 112:104958. https://doi.org/10.1016/j.bioorg.2021.104958
Supuran C, Casini A, Scozzafava A (2003) Protease inhibitors of the sulfonamidetype: anticancer anticancer, antiinlammatory, and antiviral agents. Med Res Rev 23:535–558. https://doi.org/10.1002/med.10047
Moskalik M (2023) Sulfonamides with heterocyclic periphery as antiviral agents. Molecules 28:1–15. https://doi.org/10.3390/molecules28010051
Ulusoy N (2002) Synthesis and antituberculosis activity of cycloalkylidenehydrazide and 4-aza-1-thiaspiro[4.5]decan-3-one derivatives of imidazo[2,1-b]thiazole. Arzneimittelforschung 52(7):565–571. https://doi.org/10.1055/s-0031-1299931
Cihan-Üstündağ G, Capan G (2012) Synthesis and evaluation of functionalized indoles as antimycobacterial and anticancer agents. Mol Divers 16(3):525–539. https://doi.org/10.1007/s11030-012-9385-y
Vrijens P, Noppen S, Boogaerts T, Vanstreels E, Ronca R, Chiodelli P, Laporte M, Vanderlinden E, Liekens S, Stevaert A, Naesens L (2019) Influenza virus entry via the GM3 ganglioside-mediated platelet-derived growth factor receptor beta signalling pathway. J Gen Virol 100:583–601. https://doi.org/10.1099/jgv.0.001235
Acknowledgements
This study was funded by Scientific Research Projects Coordination Unit of Istanbul University (Project Number TSA-2020-34930). L.N. acknowledges excellent technical assistance from Ria Van Berwaer and the team of Leentje Persoons.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Apaydın and Naesens wrote the main manuscript, Apaydın prepared figures and tables and Üstündağ reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Apaydın, Ç.B., Naesens, L. & Cihan-Üstündağ, G. One-pot synthesis, characterization and antiviral properties of new benzenesulfonamide-based spirothiazolidinones. Mol Divers (2024). https://doi.org/10.1007/s11030-024-10912-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-024-10912-x