Abstract
Six known products (4–9) were prepared from reaction of adipoyl chloride with 1,2,3-trimethoxybenzene according to the literature. From (2,3,4-trimethoxyphenyl)(2-(2,3,4-trimethoxyphenyl)cyclopent-1-en-1-yl)methanone (4) of them, four new 1,2-disubstituted cyclopentane derivatives (10–13) with phenyl and benzyl units were synthesized by reactions such as hydrazonation, catalytic hydrogenation and bromination. The obtained compounds 4–13 were examined for their in vitro inhibitory activity against acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and α-glucosidase enzymes. All compounds 4–13 showed inhibition at nanomolar level with Ki values in the range of 45.53 ± 7.35–631.96 ± 18.88 nM for AChE, 84.30 ± 9.92–622.10 ± 35.14 nM for BChE, and 25.47 ± 4.46–48.87 ± 7.33 for α-Glu. In silico molecular docking studies of the potent compounds were performed in the active sites of AChE (PDB: 1E66), BChE (PDB: 1P0I), and α-glucosidase (PDB: 5ZCC) to compare the effect of bromine atom on the inhibition mechanism. The optimized molecular structures, HOMO–LUMO energies and molecular electrostatic potential maps for the compounds were calculated by using density functional theory with B3LYP/6–31 + G(d,p).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phenol derivatives such as bromophenols exhibit biological activities such as antioxidant, carbonic anhydrase, α-glucosidase, α-amylase, and aldose reductase inhibitory activities. There are natural products in the phenol and bromophenol classes and they also show important biological activity [1,2,3,4,5,6]. In addition to the biological activities of these types of compounds, since they have electron-rich aromatic rings, they undergo reactions, especially electrophilic aromatic substitution reactions, and new derivatives are obtained. The structures and properties of these types of compounds are also studied mechanistically and theoretically. At different times before, we synthesized compounds with 1–3 structures formed by the reaction of benzene derivatives with methoxy groups at different positions with adipoyl chloride (Fig. 1). These compounds were published along with their biological properties [3, 7,8,9].
Alzheimer's disease (AD) is a neurological condition that affects older adults and is irreversible. The illness results in daily cognitive impairment, such as dementia and memory loss. The overproduction of the β-amyloid peptide, environmental variables, reactive oxygen species, and cholinergic insufficiency are some of the elements that affect how AD develops [10, 11]. Additionally, some metabolic enzymes, such as glycogen synthase kinase 3, acetylcholinesterase (AChE), butyrylcholinesterase (BChE), secretase, and cyclin-dependent kinase 5, as well as the N-methyl-D-aspartate receptor, play a key role in the progression of this disease. Cholinesterase inhibitors make up the majority of anti-Alzheimer's disease (AD) medications currently approved by the US Food and Drug Administration (FDA) [12]. The amount of the neurotransmitter acetylcholine in the brain is decreased as a result of cholinesterase enzymes (ChEs) hydrolyzing it more quickly. Cholinesterase inhibition thereby improves cholinergic neurotransmission.
The metabolism of carbohydrates depends on the enzyme α-glucosidase. By transforming starch and disaccharides into soluble monosaccharides like glucose, it elevates blood glucose levels in animals. Because of this activity, α-glucosidase has been suggested as a potential therapeutic target for the treatment of type-2 diabetes in people. Inhibition of this enzyme may reduce postprandial plasma glucose levels by delaying the digestion of carbohydrates and consequently the absorption of monosaccharides [13, 14]. Voglibose, acarbose, and miglitol, three often prescribed α-glucosidase inhibitors, have been linked to adverse effects include bloating, flatulence, diarrhea, soreness, and abdominal discomfort. Recently, it was discovered that bromophenols from marine algae serve as both protein tyrosine phosphatase (PTP1B) and α-glucosidase inhibitors [15,16,17].
In our previous studies, we synthesized some derivatives of compounds 1–3, and reported their biological activities such as carbonic anhydrase [3] and antioxidant [8]. In one of our recent articles [7], compounds 4–9 with OMe groups at positions 2, 3, and 4 on the phenyl rings of structures 1–3 were also obtained from the reaction of adipoyl chloride with 1,2,3-trimethoxybenzene (Fig. 2). In the study, DFT calculations including two hybrid functionals, B3LYP and M06-2X, for compounds 4–9 were performed [7]. The important experimental findings obtained in the 1H and 13C NMR data were reported by comparing them with computational data. Among compounds 4–9, (2,3,4-trimethoxyphenyl)(2-(2,3,4-trimethoxyphenyl)cyclopent-1-ene-1-yl)methanone (4) and its derivative 5 are an important compound because they have different functional groups. For further reactions, the compound 4 was chosen due to its higher proportion than compound 5 in the reaction. Therefore, reactions such as hydrazonation, catalytic hydrogenation, and bromination reactions of 4 may be formed important products because the groups in the molecule, especially the non-aromatic double bonds, can interact and compounds with different functional groups can be formed. For this purpose, the reactions of 4 have been performed, and four new products have been obtained from these reactions. Moreover, DFT studies were investigated for four new products 10–13 to see if there was a connection between the properties of the compounds. In vitro inhibitory activities against AChE, α-glucosidase, and BChE have been investigated for compounds 4–13 because phenol and phenol derivative compounds show biological activity. In silico studies have been carried out for the most biologically active compounds. Also, when we examine the kinds of literature [18,19,20], there are studies similar to the compounds that we studied, so we examined the effects of novel compounds on some metabolic enzymes and added the results of the best enzymes to our study and then proved the results with another parameter such as molecular docking. For example, In the study of Bayrak et al. [20] every synthetic substance showed a significant inhibitory effect on both cholinergic enzymes. Lineweaver–Burk graphs were created in order to determine the Ki values of novel bromophenols. The range of Ki values for AChE, BChE, and α-glycosidase was determined to be 0.13–14.74 nM, 5.11–23.95 nM, and 63.96–206.78 nM, respectively. When compared to positive controls, all bromophenols and their derivatives show an effective inhibitory profile.
Compounds 4–9 synthesized in our previous study [7]
Experimental section
General information
The values of all chemicals, solvents, and silica gels (for column and thin layer chromatography), as well as measurements of Mp of all compounds used in this work, were obtained as described previously [7, 8].
Synthesis
Synthesis of compounds 4–9 was given in the literature [7].
Synthesis of compound 10
Similar to the literature [3, 21], to a solution of compound 4 (0.1 g, 0.23 mmol) in acetic acid (15 mL) was added hydrazine hydrate (180 mg, 5.63 mmol) and the mixture was refluxed for 2 h. As a result of TLC, it was observed that the reaction was completed. After the reaction mixture cooled, it was poured into a mixture of water and ice (150 g). The mixture was extracted by CH2Cl2 (3 × 50 mL). Combined organic phases were dried over Na2SO4 and filtered and the solvent was removed in the evaporator. The residue was submitted to silica gel (20 g) column chromatography with EtOAc/hexane (2/3) elution. Product 10 (60 mg, 53%, pale yellow liquid) was obtained.
(Z)-N'-((2,3,4-Trimethoxyphenyl)(2-(2,3,4-trimethoxyphenyl)cyclopent-1-en-1-yl)methylene)acetohydrazide (10)
1H NMR (400 MHz—CDCl3): 8.20 (s, 1 NH), 6.49 (d, A part of system AB, J = 8.50 Hz, aromatic, 1H), 6.48 (d, A part of system AB, J = 8.50 Hz, aromatic, 1H), 6.36 (d, B part of system AB, J = 8.50 Hz, aromatic, 1H), 6.31 (d, B part of system AB, J = 8.50 Hz, aromatic, 1H), 3.83 (s, OCH3, 3H), 3.80 (s, OCH3, 3H), 3.76 (s, OCH3, 6H), 3.75 (s, OCH3, 6H), 2.89 (t, J = 7.60 Hz, aliphatic, 2H), 2.76 (t, J = 7.20 Hz, aliphatic, 2H), 2.21 (s, CH3, 3H), 2.04–1.95 (m, aliphatic, 2H); 13C NMR (100 MHz, CDCl3): 172.7 (CO), 154.5 (C=N), 152.4 (C), 150.5 (C), 150.3 (C), 147.2 (C), 143.8 (C), 141.7 (C), 141.4 (C), 136.8 (C), 125.4 (C), 124.0 (CH), 123.8 (CH), 118.0 (C), 107.3 (CH), 106.5 (CH), 60.8 (OCH3), 60.7 (OCH3), 60.6 (OCH3), 60.5 (OCH3), 56.0 (OCH3), 55.96 (OCH3), 41.2 (CH2), 35.3 (CH2), 22.0 (CH2), 20.4 (CH3); IR (CH2Cl2, cm−1): 3314, 2936, 2842, 1680, 1597, 1494, 1464, 1412, 1363, 1320, 1294, 1234, 1211, 1169, 1100, 1056, 1016, 984, 947, 914, 875, 796, 735, 691, 619, 553, 534. HRMS (ESI) m/z: [M + H]+ Calcd for C26H33N2O7 485.2288; Found 485.2281.
Synthesis of compound 11
A solution of compound 4 (90 mg, 0.21 mmol) in CH2Cl2 (20 mL) at room temperature in the presence of Pd/C was prepared according to the known catalytic hydrogenation procedure [3, 9, 22,23,24]. The reaction of this solution with hydrogen started and it was completed within 4 h according to thin layer chromatography (TLC). After the catalyst was removed by filtration and the solvent in the evaporator, product 11 (85 mg, 97%, colorless liquid) was obtained as the single product.
1,2,3-Trimethoxy-4-(2-(2,3,4-trimethoxybenzyl)cyclopentyl)benzene (11)
1H NMR (400 MHz, CDCl3): 6.92 (d, A part of system AB, J = 8.40 Hz, aromatic, 1H), 6.66 (d, A part of system AB, J = 8.48 Hz, aromatic, 1H), 6.64 (d, B part of system AB, J = 8.65 Hz, aromatic, 1H), 6.52 (d, B part of system AB, J = 8.40 Hz, aromatic, 1H), 3.91 (s, OCH3, 3H), 3.87 (s, OCH3, 3H), 3.85 (s, OCH3, 3H), 3.82 (s, OCH3, 3H), 3.80 (s, OCH3, 3H), 3.74 (s, OCH3, 3H), 3.56–3.48 (m, aliphatic, 1H), 2.58–2.48 (m, aliphatic, 1H), 2.21 (dd, aliphatic, J = 13.53, 3.98 Hz, 1H), 2.02–1.80 (m, aliphatic, 4H), 1.76–1.56 (m, aliphatic, 2H), 1.52–1.43 (m, aliphatic, 1H); 13C NMR (100 MHz, CDCl3): 152.3 (C), 152.1 (C), 151.8 (C), 151.6 (C), 142.17 (C), 142.15 (C), 129.1 (C), 128.2 (C), 124.3 (CH), 122.6 (CH), 106.9 (CH), 106.6 (CH), 60.8 (OCH3), 60.65 (2 OCH3), 60.60 (OCH3), 55.97 (OCH3), 55.94 (OCH3), 43.2 (CH), 42.1 (CH), 30.9 (CH2), 30.5 (CH2), 29.7 (CH2), 23.3 (CH2). IR (CH2Cl2, cm−1): 2941, 2872, 2835, 1736, 1601, 1494, 1465, 1435, 1416, 1346, 1273, 1232, 1199, 1164, 1138, 1045, 1019, 973, 941, 906, 798, 752, 737, 689; HRMS (ESI) m/z: [M + H]+ Calcd for C24H33O6 417.2277; Found 417.2268.
Bromination of compound 11
A solution of excess Br2 (1.0 mL) was added to a solution of compound 11 (0.1 g, 0.24 mmol) and CH2Cl2 (20 mL) was added dropwise, over 10 min, with stirring, at RT. After stirring at the same temperature for 20 min, Br2 and the solvent in the reaction mixture were removed by rotoevaporation. Chromatography of the crude products on silica gel (40 g) by eluting with CH2Cl2 gave tetrabromide 12 (80 mg, 46%, colorless liquid) and tribromide 13 (45 mg, 29%, colorless liquid), respectively.
1,2-Dibromo-3-(2-(2,3-dibromo-4,5,6-trimethoxybenzyl)cyclopentyl)-4,5,6-trimethoxybenzene (12)
1H NMR (400 MHz, CDCl3): 4.04–3.97 (m, aliphatic, 1H), 3.97 (s, OCH3, 3H), 3.90 (s, OCH3, 3H), 3.87 (s, OCH3, 6H), 3.85 (s, OCH3, 3H), 3.78 (s, OCH3, 3H), 2.79 (t, J = 12.19 Hz, aliphatic, 1H), 2.63–2.56 (m, aliphatic, 1H), 2.31–2.21 (m, aliphatic, 2H), 2.01–1.90 (m, aliphatic, 2H), 1.80–1.71 (m, aliphatic, 1H), 1.60–1.45 (m, aliphatic, 2H); 13C NMR (100 MHz, CDCl3): 153.3 (C), 151.9 (C), 150.6 (C), 150.2 (C), 146.6 (C), 146.4 (C), 133.84 (C), 133.80 (C), 123.6 (C), 121.5 (C), 116.0 (C), 115.82 (C), 61.0 (OCH3), 60.91 (OCH3), 60.90 (OCH3), 60.88 (2 OCH3), 60.7 (OCH3), 49.4 (CH), 43.0 (CH), 33.1 (CH2), 31.0 (CH2), 30.4 (CH2), 25.6 (CH2); IR (CH2Cl2, cm−1): 2937, 2866, 1562, 1460, 1403, 1392, 1352, 1296, 1265, 1237, 1195, 1138, 1101, 1049, 1007, 962, 740, 705; HRMS (APCI) m/z: [M + Na]+ Calcd for C24H28Br4NaO6 750.8517; Found 750.8512.
1,2-Dibromo-3-((2-(5-bromo-2,3,4-trimethoxyphenyl)cyclopentyl)methyl)-4,5,6-trimethoxybenzene (13)
1H NMR (400 MHz, CDCl3): 7.17 (s, aromatic, 1H), 3.92 (s, OCH3, 6H), 3.90 (s, OCH3, 3H), 3.87 (s, OCH3, 3H), 3.85 (s, OCH3, 3H), 3.75 (s, OCH3, 3H), 3.54 (dd, J = 15.66, 8.03 Hz, aliphatic, 1H), 2.63–2.56 (m, aliphatic, 1H), 2.49 (t, J = 12.3 Hz, aliphatic, 1H), 2.27 (dd, J = 12.57, 3.34 Hz, 1H), 1.97–1.93 (m, aliphatic, 3H), 1.69–1.54 (m, aliphatic, 3H); 13C-NMR (100 MHz, CDCl3): 152.0 (2C), 150.2 (C), 149.3 (C), 147.2 (C), 146.3 (C), 133.6 (C), 133.2 (C), 126.3 (CH), 121.5 (C), 116.0 (C), 110.7 (C), 61.0 (OCH3), 60.94 (OCH3), 60.91 (OCH3), 60.90 (OCH3), 60.86 (OCH3), 60.84 (OCH3), 42.4 (CH), 42.2 (CH), 32.3 (CH2), 29.49 (CH2), 29.47 (CH2), 23.1 (CH2); IR (CH2Cl2, cm−1): 3051, 2937, 2870, 1732, 1564, 1461, 1421, 1402, 1348, 1294, 1266, 1195, 1148, 1097, 1078, 1049, 1008, 961, 929, 866, 820, 792, 739, 705; HRMS (APCI) m/z: [M + H]+ Calcd for C24H30Br3O6 652.9572; Found 652.9591.
Biological assays
The Ellman [25] approach was employed to examine the impact of synthetic chemicals on the ability to inhibit AChE and BChE activity. AChI/BChI and DTNB were used to quantify the AChE/BChE activity. The sources of the enzymes we studied in this article are as follows: Acetylcholinesterase from Electrophorus electricus (electric eel, CAS No.: 9000-81-1), Butyrylcholinesterase from equine serum (CAS No.: 9001–08-5), and α-Glucosidase from Saccharomyces cerevisiae (CAS Number: 9001-42-7). For different concentrations of the sample solution, 100 μL of buffer (1 M, Tris/HCl, pH 8.0) were used to dissolve 10 μL of the sample solution. 50 μL were incubated at 25 °C for 10 min after the AChE/BChE (5.32103 EU) solution was added. The incubation was followed by the addition of DTNB (50 μL, 0.5 mM) [26,27,28]. Lastly, to start the reaction, 50 μL of bChI/aChI were added. The enzymatic hydrolysis of both substrate molecules was obtained by measuring the spectrophotometric production of the 5-thio-2-nitrobenzoate molecule at a wavelength of 412 nm as an impact of the reaction of DTNB molecule with thiocholine molecule. All compounds 4–13 were incorporated into the reaction mixture in various dosages to see how they would affect AChE. Then, the activities of AChE/BChE were evaluated [29].
According to Tao et al.’s method [30], the activity of synthesized compounds 4–13 on α-glucosidase was measured using the substrate p-nitrophenyl-α-D-glucopyranoside (p-NPG). Samples were made by dissolving 20 mg in 20 mL (EtOH:H2O). It was initially combined in 5–200 μL of sample with 700 μL of phosphate buffer (0.15 u/mL, pH 7.4) and 20 μL of enzyme solution [31, 32]. After a pre-incubation period of 10 min at 35 °C, 50 μL of p-NPG was added as the reaction began. Additionally, following the pre-incubation, 50 μL of p-NPG in phosphate buffer (5 mM, pH = 7.4) was added, and the incubation was repeated at 35 °C. A curve was fitted to the data to determine the IC50 and Ki values. The chemical acarbose was used as a positive control. At 405 nm, absorbances were spectrophotometrically quantified [33]. One mole of substrate is hydrolyzed at a rate of one unit of α-glucosidase per minute (pH: 7.4) [34].
Molecular docking protocol
Molecular docking studies are commonly carried out to predict the binding interaction energies of the identified ligands with the selected proteins. In molecular docking calculations, the most potent active ligands were optimized with the DFT/B3LYP method and 6–31 + G(d,p) basis using the software Gaussian 09W. The 3D crystal structures of the three selected enzymes were downloaded from the Protein Data Bank (PDB) (https://www.rcsb.org/) as follows: 1E66 for AChE [35], 1P0I for BChE [36], and 5ZCC for α-glucosidase [37]. The hetero groups (other ligands, ions, and water) of the proteins were removed from the PDB formats of the protein structures. The in silico docking analyses were performed on AChE, BChE, and α-glucosidase via AutoDock Vina v.1.5.7 [38]. The grid parameters determined were 80 × 70 × 64 Å3 x, y, z dimensions, 1.000 Å space, and 5.085, 65.254, 56.371 x, y, z centers for AChE (PDB: 1E66); 68 × 78 × 76 Å3 x, y, z dimensions, 0.375 Å space, and 131.841, 116.094, 38.717 x, y, z centers for BChE (PDB: 1P0I); and 66 × 56 × 90 Å3 x, y, z dimensions, 0.825 Å space, and 3.195, 48.279, 82.191 x, y, z centers for α-glucosidase (PDB: 5ZCC) as active residues of the target proteins. According to the results for biological activity, the best positions of the selected compounds 9, 12, and 13 were visualized and evaluated using BIOVIA Discovery Studio Visualizer v21.1.0.20298 (https://www.3dsbiovia.com/) and polar hydrogens added.
Results and discussion
Synthesis
Known compounds 4–9 (Fig. 2) were synthesized from the reaction of 1,2,3-trimethoxybenzene with adipoyl chloride in the presence of AlCl3 [7]. Compound 4 is an α,β-unsaturated compound including two benzene rings with trimethoxy groups. Important compounds may be obtained from 4 because it has functional groups such as α,β-unsaturated ketone and benzoyl and phenyl groups with OMe. The compounds to be obtained can be target compounds or intermediates (especially bromides). Refluxing compound 4 with hydrazine monohydride (NH2NH2.H2O) in acetic acid (HOAc) gave a compound that also contained the acetyl group (Scheme 1). This compound should be a hydrazone derivative as 10 because its NMR spectra show an NH hydrogen (at 8.20 ppm as a singlet) and six aliphatic hydrogens (at 2.89–1.95 ppm) in the 1H NMR spectrum and sixteen C lines in the double bond region (172.73–106.48 ppm) in the 13C NMR spectrum. The reaction of an α,β-unsaturated ketone with hydrazine gives pyrazoline derivatives [3, 21, 39,40,41]. The inability to observe a pyrazoline derivative instead of a hydrazone derivative in the reaction of compound 4 with hydrazine monohydride may be due to the OMe in the o-position of the phenyl ring attached to the β carbon atom of 4. The structure of hydrazone 10 can be either trans- or cis-structure. As shown Scheme 1, this structure was assumed to be in a trans structure, which would be more stable when steric effects are taken into account.
A catalytic hydrogenation reaction (H2, Pd/C) of compound 4 was performed, and one product was isolated from this reaction. It is known that in the reduction of α,β-unsaturated ketones such as 4, first double bound group, and then the carbonyl group are reduced [3]. It is very difficult to determine the configurations of the vicinal bulky (substituted benzyl and phenyl) groups in the quintuple ring in this product by NMR spectroscopy. Having obtained a single crystal of this product, its structure is easy to explain by X-ray analysis. However, we were unable to obtain its crystal. Double bonds are reduced in the cis-configuration by catalytic hydrogenation. A trans-isomer was obtained in the reduction of the cyclohexene derivative compound by catalytic hydrogenation [42]. In the catalytic hydrogenation reaction of 4, the reduction product was assumed to be 11 due to the highly steric effect.
The reaction of compound 11 with molecular bromine performed, and two products were obtained from the chromatography of crude products on silica gel. When the 1H NMR spectra of these two products were examined, it was observed that one of them had no aromatic hydrogen peak while the other one had an aromatic hydrogen peak (at 7.17 ppm as s). The product without one aromatic hydrogen should be tetrabromide 12 and the other a tribromide compound. It was accepted that the structure of the tribromide was 13 and the steric state was effective in its formation. 1H NMR spectrum of the tetrabromide 12 is consistent with the proposed structure. However, we had a doubt as to whether the peak at 49.4 ppm in the 13C NMR spectrum belongs to the compound 12. To clarify this, the HMQC spectrum of the compound was taken. Due to the slow rotation, the small and broad-looking peak should be the benzylic CH adjacent to the phenyl ring. The HMQC spectrum of the compound 12 is given in the Supporting Information file.
Biological activity
In the present study, preparated compounds were evaluated against some metabolic enzymes (α-Glu, AChE, and BChE) (Table 1).
-
1.
Long-chain dietary carbohydrates are hydrolyzed by the enzyme α-glucosidase in the small intestine to form monosaccharide units that enter the bloodstream and cause hyperglycemia [43]. As a result, α-glucosidase inhibition has become a crucial therapeutic target with the potential to lower blood sugar levels by slowing down the digestion of carbohydrates [44]. The α-glucosidase inhibitors (Fig. 3), however, primarily influence hyperglycemia without directly influencing insulin secretion [45]. The data obtained demonstrated that some of the preparated compounds with Ki values ranging from 25.47 ± 4.46 to 48.87 ± 7.33 nM exhibited inhibitory activity more than the positive control acarbose, with a Ki value of 43.06 ± 6.07 nM for α-glucosidase Among the compounds synthesized, the most potent were 9, 12, and 6, with Ki values of 25.47 ± 4.46, 27.42 ± 5.17, and 29.13 ± 4.25 nM, respectively. These compounds were 1- to 1.5-fold more potent than the positive control acarbose. On the other hand, compounds 13, 11, and 8 showed average and near inhibition potentials, with Ki values of 35.63 ± 3.95, 38.14 ± 5.48, and 38.51 ± 4.25 nM, respectively, against this enzyme. Additionally, the Ki results for compounds 5, 7, and 4 (44.71 ± 6.61, 46.47 ± 6.34, and 48.87 ± 7.33 nM, respectively) were similar to those for the standard (Ki: 43.06 ± 6.07 nM). As a result, they are utilized as monotherapy in the treatment of moderate diabetes and are regarded as the first-line oral sugar-reducing medications. In cases of acute diabetes, they are utilized in combination with insulin or other drugs [46]. However, research has found that these drugs also have a number of side effects, including diarrhea, abdominal pain, bloating, and flatulence. These factors have led researchers to concentrate on discovering novel substances and, in recent years, a large number of novel substances have been identified as α-glucosidase inhibitors [47]. The small intestine's enzyme α-glucosidase has the ability to hydrolyze the α − 1 \(\dot{ \to }\) 4 bond of oligosaccharides or disaccharides, resulting in the production of glucose. Evidently, the conversion of carbohydrates into blood glucose might be significantly inhibited by inhibiting the activity of α-glucosidase. As a result, the reduced adsorption of glucose could lower the postprandial blood glucose level, which is a useful way to lessen postprandial hyperglycemia symptoms in T2DM patients [40, 45]. Indeed, α-glucosidase inhibitors can be candidates for anti-diabetic drugs in living cells; therefore, we detected the compounds in the present study as new inhibitors in vitro (Fig. 2). We intend to study these inhibitors in vivo in our future studies.
-
2.
The AChE inhibitors donepezil and galantamine, as well as a dual inhibitor called rivastigmine, are currently the only anticholinesterase medications that the FDA has approved. They have a positive impact on the cognitive, functional, and behavioral signs and symptoms of the condition [48]. Although these medications are generally beneficial, several side effects have been noted, including exhaustion, nausea, vomiting, muscle cramps, diarrhea, and loss of appetite. In this regard, much effort has been made to create novel dual cholinesterase inhibitors for the treatment of AD’s neurological problems. Finding new candidate medications that target various AD-related enzymes by combining in vitro and in silico research represents a valuable strategy [49, 50]. The Ki values of the test compounds demonstrated that the most active compounds against AChE were 9, 12, and 5 (Ki: 45.53 ± 7.35, 61.15 ± 5.26, and 87.91 ± 7.27 nM, respectively) (Table 1). Moreover, for AChE, the IC50 values of tacrine (TAC) as the positive control and the novel compounds examined in the present study were in the following order: 9 (49.84 nM, r2: 0.9391) < 12 (65.52 nM, r2: 0.9850) < 5 (92.25 nM, r2: 0.9550) < 4 (102.32 nM, r2: 0.9891) < 13 (124.16 nM, r2: 0.9943) < TAC (241.61 nM, r2: 0.9366). The most potent compounds among the synthesized derivatives against BChE were 12, 10, and 11 with Ki values of 84.30 ± 9.92, 131.22 ± 14.54, and 144.63 ± 15.46 nM, respectively. The data obtained demonstrated that the synthesized compounds, with Ki values ranging from 84.30 ± 9.92 to 622.10 ± 35.14 nM, exhibited greater inhibitory activity than the positive control tacrine, with a Ki value of 352.15 ± 23.55 nM, BChE except for compounds 4, 7, and 9 (Table 1). Additionally, for AChE, the Ki values of tacrine (TAC) as the positive control and some compounds examined in the present study were in the following order: 9 (45.53 ± 7.35 nM) < 12 (61.15 ± 5.26 nM) < 5 (87.91 ± 7.27 nM) < 4 (99.72 ± 11.44 nM) < 13 (108.14 ± 5.14 nM) < TAC (226.52 ± 19.43 nM). Moreover, for BChE, the Ki values of tacrine (TAC) as the positive control and some compounds examined in the present study were in the following order: 12 (84.30 ± 9.92 nM) < 10 (131.22 ± 14.54 nM) < 11 (144.63 ± 15.46 nM) < 5 (175.62 ± 23.80 nM) < 13 (205.13 ± 17.41 nM) < TAC (352.15 ± 23.55 nM). Also, for α-Glu, the Ki values of ACR as the positive control and some compounds examined in the present study were in the following order: 9 (25.47 ± 4.46 nM) < 12 (27.42 ± 5.17 nM) < 6 (29.13 ± 4.25 nM) < 10 (31.23 ± 5.54 nM) < 13 (35.63 ± 3.95 nM) < ACR (43.06 ± 6.07 nM).
Molecular docking studies
Molecular docking simulations were performed to predict possible interactions between the most active compounds among the preparated compounds 4–13 and the active sites of the three enzymes (AChE, BChE, and α-glucosidase). In this study, in vitro and docking studies were also carried out by adding compounds 4–9, including the derivatives of these compounds, in addition to the new compounds 10–13. According to the results of the in vitro analysis, tetrabromide 9 showed a stronger inhibitory activity for both AChE and α-glucosidase target enzymes than the control molecule, tacrine (Table 1). On the other hand, tetrabromide 12 showed the strongest activity for the BChE target enzyme, while it showed the second strongest inhibitory activity for both AChE and α-glucosidase. What is surprising here is that tribromide 13, which is in the structure of tetrabromide 12 and has only one less bromine atom, has much less activity than tetrabromides 9 and 12 with respect to Ki values (Table 1). Therefore, the interactions between 9, 12, and 13 with α-glucosidase (PDB: 5ZCC) [37] were simulated to examine the effect of bromine atom on the inhibition mechanism. Moreover, in silico molecular docking analyses of tetrabromide 9 with AChE (PDB: 1E66) [35] and of tetrabromide 12 with BChE (PDB: 1P0I) [36] receptors were conducted.
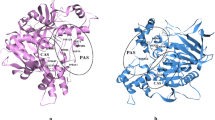
The three-dimensional (3D) and two-dimensional (2D) graphs obtained for docked 9, 12, and 13 into the active site of α-glucosidase by considering the best bonding results are shown in Fig. 4. For 9, 12, and 13 with α-glucosidase (PDB: 5ZCC), the molecular docking scores (the best binding energy values) calculated were − 6.5, − 6.4, and − 6.1 kcal/mol, respectively. As can be clearly seen in Fig. 4, the higher activity of 9 compared to 12 and 13 mainly refers to the number of both hydrogen bonds and other interactions formed in the 9-α-glucosidase complex. Two conventional hydrogen bonds and two carbon hydrogen bonds are formed between 9 and amino acid residues of α-glucosidase active sites Gln328, Arg411 and Gly384, Ile143, respectively. Compound 9 forms one strong hydrogen bond between the oxygen atom of the carbonyl group and Gln328 of length 1.99 Å. The second hydrogen bond is formed between the oxygen atom of the methoxy group and Arg411 of length 2.92 Å. The other five non-hydrogen bonding interactions are of type π-anion, alkyl, and π-alkyl formed with the amino acid residues Asp327, His203, Phe163, Ile143, and Phe225.
In compound 12, the five carbon hydrogen bonds occurred between Glu271 and C36 of length 3.29 Å, between Glu271 and Br60 of length 3.52 Å, between Ala247 and C48 of length 3.66 Å, between Ala247 and C44 of length 3.30 Å, and between Lys242 and O25 of length 350 Å. The other interactions, which are alkyl and π-alkyl type interactions were observed as shown in Fig. 4. These four non-hydrogen bonding interactions are of alkyl and π-alkyl types, formed with the amino acid residues Trp6, Lys242, and Ala247 (Fig. 4).
Furthermore, in compound 13, one of the two hydrogen bonds, of length 1.81 Å, is formed between the oxygen atom of the methoxy group and the amino acid residue Trp288, while the other, of length 2.28 Å, is formed between the oxygen atom of the methoxy group and the amino acid residue Gln328. The five carbon hydrogen bonding interactions are also formed between Ile143 and C44 of length 3.31 Å, between Ser145 and C44 of length 3.31 Å, between Gly384 and C44 of length 3.40 Å, between Asp382 and C40 of length 3.41 Å, and between Gly384 and O25 of length 3.70 Å (Fig. 5). As a result, as seen in Fig. 4, in this section where we examined the effect of the bromine atom on the binding, while there were four and two interactions between the bromine atoms in tetrabromides 9 and 12 and the amino acid residues of α-glucosidase, respectively, no interaction was observed with the bromine atoms in tribromide 13. While compounds 9 and 12, which contain four bromine atoms, show a very strong activity against α-glucosidase, it is interesting that compound 13 which has only one atom missing shows a weak activity despite having the same skeletal structure as compound 12. This shows that the number of bromine atoms in the molecule has an effect on the inhibition activity.
For compound 9, showing the strongest activity against AChE (PDB: 1E66), the best binding energy value calculated was − 6.5 kcal/mol. Compound 9 forms one strong hydrogen bond between the hydrogen (H30) of the hydroxyl group and the amino acid residue Gln74 of length 2.19 Å. The other interactions of 9 involving two carbon hydrogen bonds and π-π stacked interaction were formed with the amino acid residues Gln74, Phe331, and Trp279 (Fig. 5).
For compound 12, showing the strongest activity against BChE (PDB: 1P0I), the molecular docking score calculated was − 7.4 kcal/mol. Compound 12 forms a strong hydrogen bond between the oxygen atom (O24) of the methoxy group and His438 of length 2.31 Å. The other interactions of 12 involving one carbon hydrogen bond, π-π T-shaped, and π-alkyl were formed between Pro285 and C28 with 3.51 Å, between Phe329 and the phenyl ring of the benzyl group with 5.06 Å, and between His438 and Br60 with 4.96 Å, respectively (Fig. 5).
DFT studies
Molecular structure analysis
All computations were performed using the software Gaussian 09W [51]. Geometric optimizations of the compounds were performed using density functional theory method at the B3LYP with 6–31 + G(d,p) basis set [52, 53]. The results were visualized by the visualization software CYLview [54] and GaussView 5.0 [55]. DFT is an excellent method often used to describe molecular structure and stability, mechanistic insights, and molecular interactions [56,57,58,59,60,61].
In our previous study, DFT studies including structural properties, natural bond orbital analysis of donor–acceptor interactions, charges on the atoms, comparison of intramolecular hydrogen bonds, and quantum chemical reactivity identifiers for compounds 4–9 were performed using two functional levels, B3LYP and M06-2X, and examined in detail [7]. Moreover, the experimental 1H and 13C NMR chemical shifts were compared with the calculated values.
In the synthesis section, the molecular structure and total energies of compound 4 were examined in order to support that the product formed in the catalytic hydrogenation could be a trans isomer. To compare the stabilities of cis-11 and trans-11, both compounds were optimized using the B3LYP/6–31 + G(d,p) basis set (Fig. 6). The relative total energy for trans-11 carried out at the B3LYP/6–31 + G(d,p) level is lower for 2.23 kcal/mol than the energy for cis-11 (Fig. 6). The theoretical calculations support the proposed configuration of the groups in the cyclopentane ring. Compounds 10–13 were optimized at DFT/B3LYP/6–31 + G(d,p) level of theory in the gas phase (Fig. 7). Some selected structural parameters of compounds 10–13 are given in Table S1. The dihedral angles C4–C3–C9–C10 and C19–C18–C12–C10 for 10 and 11 are − 41.6° and − 76.0°, and − 52.2° and − 55.5°, respectively. According to these values, the phenyl and cyclopentene rings for compound 10 were deviated from the plane due to weak conjugation.
Natural bond orbital (NBO) analysis
NBO analysis is a useful computational approach used both to investigate intramolecular interactions, hydrogen bonds, and charge transfers and to calculate hyperconjugative interactions between atoms and molecules. A larger stabilization energy (E(2)) value indicates that there is more intense interaction between electron donors and electron acceptors, and the extend of the conjugation of the whole system is also greater. E(2) values can be calculated by second order perturbation theory using the following equation [62, 63]:
In this equation, qi is “the donor orbital occupancy”, ɛj and ɛi are diagonal elements, and (Fij) is “the off-diagonal NBO Fock matrix element”.
Selected NBO donor–acceptor interactions in the analysis results of compounds 10–13 are given in Table 2. The significant hyperconjugative interactions and the stabilization energies were observed by NBO analysis. In compound 10, strong intramolecular hydrogen bonding was observed from LP(1) N59 to antibonding orbitals σ*(N60-H61) with a stabilization energy of 9.35 kcal/mol. Moreover, considerably high hyperconjugative interaction values were observed in compounds 12 and 13 due to the bromine atoms attached to the aromatic ring. The hyperconjugative interaction energies of LP(2) O22 → σ*(C19–C21), LP(2) O25 → σ*(C19–C21), LP(1) Br62 → σ*(C10–H58) and LP(2) Br62 → σ*(C19–C21) for 12 are 1035.83, 1049.99, 3590.65 and 3621.41 kcal/mol, respectively. The hyperconjugative interaction energies of LP(2) O27 → σ*(C48-H51), LP(1) Br60 → σ*(C44-H46) and LP(1) Br60 → σ*(C48-H51) for 13 are 1266.37, 233.74 and 228.85 kcal/mol, respectively.
In addition, the NBO analysis also shows the natural charge on the atoms in the molecule. Natural charges and Mulliken atomic charges are listed in Tables S2 and S3. The charge distributions on the atoms give important information about how the charge transfers in the molecule will take place. In general, natural population analysis data for atomic charges are more preferable than Mulliken charges. In compound 10, the charge of the carbon atom (C16) with the largest negative charge is − 0.47822, while in compound 11, the charge of the carbon atom (C13) is − 0.48081. For 10, it was observed that N60 (− 0.46622) is a more electronegative atom than N59 (− 0.25729). The oxygen (O63) of the carbonyl group draws the electrons from the nitrogen atom (N60), and therefore N60 atom has a larger negative charge. The carbon atoms C4, C5, C6, C18, C20 and C21 have a positive charge in the charge allocation of compound 12 and 13, indicating that they are acceptor atoms. In both compounds, the other carbon atoms have a negative charge, and they operate as donors. In compounds 10, 11, 12 and 13, the highest positive charge for all hydrogen atoms was found as H61 (0.42659), H59 (0.26498), H58 (0.27231) and H55 (0.27231), respectively.
Frontier molecular orbital (FMO) analysis
The energy of the frontier molecular orbitals (HOMO and LUMO) is often used to make inferences about the chemical reactivity and kinetic stability of the molecules of interest. The HOMO–LUMO gap helps determine the chemical reactivity of the molecule. According to this approach, a molecule with a small boundary orbital gap becomes more polarized, facilitating the transition of electrons from HOMO to LUMO, thus having low kinetic stability and high chemical reactivity [64,65,66]. The HOMO and LUMO energies for compounds 10–13 were calculated using the DFT/B3LYP/6–31 + G(d,p) basis set. The charge density distributions of the HOMO and LUMO orbitals for compounds 10–13 are shown in Fig. 8.
It is clearly seen in Fig. 8 that, in the case of the HOMO, the charges density for compounds 10 and 11 is completely localized on almost all the molecules. In the case of the LUMO, the charge density for compound 11 is concentrated on the phenyl ring attached to the cyclopentyl ring, while the charge for compound 10 is more densely distributed on the other phenyl ring and the acetohydrazone group attached to it and the cyclopentyl ring.
Among compounds 4–13, based on the Ki values obtained, compound 9, having four bromine atoms, had the most powerful inhibition against AChE and α-glucosidase, with the Ki values of 45.53 nM and 25.47 nM, respectively. It was also observed that compound 12, the other compound with four bromine atoms, was the second most active compound against AChE and α-glucosidase. Moreover, compound 12 showed the strongest activity against BChE, with a Ki value of 84.30 nM. Compound 10, including an acetohydrazone group, was the second most active compound against BChE, with a Ki value of 131.22 nM. What is surprising here is that compound 13, which has three bromine atoms in similar positions, showed much less activity than compounds 9 and 12 for AChE, BChE, and α-glucosidase, with Ki values of 108.14, 205.13, and 35.63 nM, respectively.
To explain this situation, we examined frontier molecular orbital images (Fig. 8) and MEP surfaces (Fig. 9). The frontier molecular orbital analysis of compound 9 was performed in detail in our previous study [7]. In the case of the HOMO, the charge densities for the symmetric compound 9 spread out from the aromatic ring to the carbonyl group. In the case of the LUMO, the charges spread from the phenyl ring to the carbon atom to which the bromine atom is bonded. In the case of the HOMO, the charges for compound 12 are located on the benzyl group, while in the case of the LUMO, the charges are located on the phenyl group attached to the cyclopentene ring. In the case of the HOMO, the charges for compound 13 are mostly located on the phenyl ring containing two bromine atoms, with a very small charge on the other phenyl ring. In the case of the LUMO, the charges are spread only on the phenyl ring containing dibromine atoms.
The quantum chemical reactivity parameters are commonly used to make inferences about the chemical behavior of molecules. These parameters for 10–13 are given in Table 3. According to Koopmans’ theorem [67], the HOMO and LUMO energies are related to electron affinity (A = − LUMO) and ionization energy (I = − HOMO). Several global reactivity identifiers such as electron affinity (A), ionization potential (I), global chemical hardness (η), global softness (σ), electronegativity (χ), electrophilicity (ω), and chemical potential (μ) have been calculated using by the following equations [68,69,70].
Molecular electrostatic potential (MEP) analysis
The MEP surfaces of compounds 10–13 are illustrated in Fig. 9. MEP analysis provides visual information about determining reactive sites for electrophilic and nucleophilic attacks and three-dimensional plots of total charge density of the molecule [71]. In Fig. 9, red shows the negative side of the molecule, consisting of electron-rich regions, while blue depicts the positive side, consisting of electron-poor regions. The electron-rich red regions in 10 are mainly located around the carbonyl group of the acetohydrazide group. For compounds 10–13, the electron-rich (red) regions are mainly located around the oxygens of the methoxy groups and the bromine atoms.
Conclusions
The products 10 and 11 were obtained from the reactions of known 4 with hydrazine hydrate and catalytic hydrogenation, respectively. Bromination of 11 were performed, and tetrabromide 12 and tribromide 13 were obtained from the reaction. It should be that configurations of molecules are preserved in the products (except for 11) of reactants containing cyclopentene or cyclopentane units. The reaction products were purified and their structures interpreted. When the biological activities of the prepared compounds 4–13 are examined. Most of the prepared compounds inhibited AChE and BChE better than the positive control tacrine and α-glucosidase better than acarbose. It is a very interesting finding that the activities of the tetrabromides 9 and 12, in which each has two bromines attached to each phenyl ring, have much stronger activity than the tribrominated derivatives against AChE, BChE, and α-glucosidase. In silico molecular docking studies were also conducted on compounds 9, 12, and 13 to predict their binding modes with the active sites of the respective enzymes. Because of the interactions between the bromine atoms of tetrabromides 9 and 12, and amino acid residues of the α-glucosidase, as well as the absence of any interaction with the bromine atoms in tribromide 13, these important findings observed will also shed light on the binding mechanism of ligands to the receptor. DFT studies for compounds 10–13 were carried out for a better understanding of the potential AChE, BChE, and α-glucosidase activity mechanism.
Data availability
All data are available in the article and its supplementary material.
References
Ashby J (1996) Naturally occurring organohalogen compounds. Springer, Vienna
Gribble GW (2018) Newly discovered naturally occurring organohalogens. Arkivoc 2018:372–410. https://doi.org/10.24820/ARK.5550190.P010.610
Artunç T, Çetinkaya Y, Göçer H et al (2016) Synthesis of 4-[2-(3,4-dimethoxybenzyl)cyclopentyl]-1,2-dimethoxybenzene derivatives and evaluations of their carbonic anhydrase isoenzymes inhibitory effects. Chem Biol Drug Des 87:594–607. https://doi.org/10.1111/cbdd.12695
Çetinkaya Y, Menzek A, Şahin E, Balaydın HT (2011) Selective O-demethylation during bromination of (3,4-dimethoxyphenyl)(2,3, 4-trimethoxyphenyl)methanone. Tetrahedron 67:3483–3489. https://doi.org/10.1016/j.tet.2011.03.033
Çetinkaya Y, Göçer H, Menzek A, Gülçin I (2012) Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4- trihydroxyphenyl)methanone and its derivatives. Arch Pharm (Weinheim) 345:323–334. https://doi.org/10.1002/ardp.201100272
Taslimi P, Gülçin I, Öztaşkın N et al (2016) The effects of some bromophenols on human carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 31:603–607. https://doi.org/10.3109/14756366.2015.1054820
Çetinkaya Y, Artunç T, Menzek A (2022) AlCl3-catalyzed cascade reactions of 1,2,3-trimethoxybenzene and adipoyl chloride: spectroscopic investigations and density functional theory studies. ACS Omega 7:38882–38893. https://doi.org/10.1021/acsomega.2c04612
Artunc T, Menzek A, Taslimi P et al (2020) Synthesis and antioxidant activities of phenol derivatives from 1,6-bis(dimethoxyphenyl)hexane-1,6-dione. Bioorg Chem 100:103884. https://doi.org/10.1016/j.bioorg.2020.103884
Artunç T, Menzek A (2022) Synthesis and reactions of di(thiophen-2-yl)alkane diones: cyclocondensation. Turkish J Chem 46:1397–1404
Yiğit B, Taslimi P, Barut Celepci D et al (2023) Novel PEPPSI-type N-heterocyclic carbene palladium(II) complexes: synthesis, characterization, in silico studies and enzyme inhibitory properties against some metabolic enzymes. Inorg Chim Acta 544:121239. https://doi.org/10.1016/j.ica.2022.121239
Altay A, Yeniceri E, Taslimi P et al (2022) LC-MS/MS analysis and diverse biological activities of Hypericum scabrum L.: in vitro and in silico research. S Afr J Bot 150:940–955. https://doi.org/10.1016/j.sajb.2022.08.032
Koçyiğit ÜM, Ökten S, Çakmak O et al (2022) Arylated quinoline and tetrahydroquinolines: synthesis, characterization and their metabolic enzyme inhibitory and antimicrobial activities. ChemistrySelect 7:202203469. https://doi.org/10.1002/slct.202203469
Naseem S, Shafiq Z, Taslimi P et al (2023) Synthesis and evaluation of novel xanthene-based thiazoles as potential antidiabetic agents. Arch Pharm (Weinheim) 356:2200356. https://doi.org/10.1002/ardp.202200356
Sadeghi M, Khomartash MS, Gorgani-Firuzjaee S et al (2022) α-glucosidase inhibitory, antioxidant activity, and GC/MS analysis of Descurainia sophia methanolic extract: In vitro, in vivo, and in silico studies. Arab J Chem 15:104055. https://doi.org/10.1016/j.arabjc.2022.104055
Koren S, Fantus IG (2007) Inhibition of the protein tyrosine phosphatase PTP1B: potential therapy for obesity, insulin resistance and type-2 diabetes mellitus. Best Pract Res Clin Endocrinol Metab 21:621–640
Shi D, Xu F, He J et al (2008) Inhibition of bromophenols against PTP1B and anti-hyperglycemic effect of Rhodomela confervoides extract in diabetic rats. Chin Sci Bull 53:2476–2479. https://doi.org/10.1007/s11434-008-0353-y
Wang W, Okada Y, Shi H et al (2005) Structures and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J Nat Prod 68:620–622. https://doi.org/10.1021/np040199j
Çetinkaya Y, Göçer H, Gülçin I, Menzek A (2014) Synthesis and carbonic anhydrase isoenzymes inhibitory effects of brominated diphenylmethanone and its derivatives. Arch Pharm (Weinheim) 347:354–359. https://doi.org/10.1002/ardp.201300349
Abbasi MA, Saeed A, Rehman AU et al (2014) Synthesis of brominated 2-phenitidine derivatives as valuable inhibitors of cholinesterases for the treatment of Alzheimer’s disease. Iran J Pharm Res 13:87–94
Bayrak C, Taslimi P, Kilinc N et al (2023) Synthesis and biological activity of some bromophenols and their derivatives including natural products. Chem Biodivers 20:e202300469. https://doi.org/10.1002/cbdv.202300469
Asad M, Khan SA, Arshad MN et al (2021) Design and synthesis of novel pyrazoline derivatives for their spectroscopic, single crystal X-ray and biological studies. J Mol Struct 1234:130131. https://doi.org/10.1016/j.molstruc.2021.130131
Akbulut N, Balci M (1988) A new and stereospecific synthesis of cyclitols: (1,2,4/3)-, (1,2/3,4)-, and (1,3/2,4)-cyclohexanetetrols. J Org Chem 53:3338–3342. https://doi.org/10.1021/jo00249a039
Menzek A, Gökmen M (2002) Synthesis and rearrangement reactions of dihydrobenzhomobarrelene derivatives: Influence of double bond on product distribution. J Chem Res - Part S. https://doi.org/10.3184/030823402103170637
Menzek A, Altundaş A (2006) Reactions of 3,10-epoxycyclo[10.2.2.02,11.04,9]hexadeca-4,6,8,13-tetraene: a new intramolecular 1,5-oxygen migration. Tetrahedron 62:12318–12325. https://doi.org/10.1016/j.tet.2006.10.012
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Hashmi S, Khan S, Shafiq Z et al (2021) Probing 4-(diethylamino)-salicylaldehyde-based thiosemicarbazones as multi-target directed ligands against cholinesterases, carbonic anhydrases and α-glycosidase enzymes. Bioorg Chem 107:104554. https://doi.org/10.1016/j.bioorg.2020.104554
Daşgın S, Gök Y, Barut Celepci D et al (2021) Synthesis, characterization, crystal structure and bioactivity properties of the benzimidazole-functionalized PEPPSI type of Pd(II)NHC complexes. J Mol Struct 1228:129442. https://doi.org/10.1016/j.molstruc.2020.129442
Bal S, Demirci Ö, Şen B et al (2021) PEPPSI type Pd(II)NHC complexes bearing chloro-/fluorobenzyl group: synthesis, characterization, crystal structures, α-glycosidase and acetylcholinesterase inhibitory properties. Polyhedron 198:115060. https://doi.org/10.1016/j.poly.2021.115060
Akocak S, Taslimi P, Lolak N et al (2021) Synthesis, characterization, and inhibition study of novel substituted phenylureido sulfaguanidine derivatives as α-glycosidase and cholinesterase inhibitors. Chem Biodivers 18:2000958. https://doi.org/10.1002/cbdv.202000958
Tao Y, Zhang Y, Cheng Y, Wang Y (2013) Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed Chromatogr 27:148–155. https://doi.org/10.1002/bmc.2761
Tokalı FS, Taslimi P, Demircioğlu İH et al (2021) Design, synthesis, molecular docking, and some metabolic enzyme inhibition properties of novel quinazolinone derivatives. Arch Pharm (Weinheim) 354:2000455. https://doi.org/10.1002/ardp.202000455
Sertçelik M, Öztürkkan Özbek FE, Taslimi P et al (2021) Supramolecular complexes of Ni (II) and Co (II) 4-aminobenzoate with 3-cyanopyridine: synthesis, spectroscopic characterization, crystal structure, and enzyme inhibitory properties. Appl Organomet Chem 35:6182. https://doi.org/10.1002/aoc.6182
Mirzazadeh R, Asgari MS, Barzegari E et al (2021) New quinoxalin-1,3,4-oxadiazole derivatives: synthesis, characterization, in vitro biological evaluations, and molecular modeling studies. Arch Pharm (Weinheim) 354:2000471. https://doi.org/10.1002/ardp.202000471
Bal S, Demirci Ö, Şen B et al (2021) Silver N-heterocyclic carbene complexes bearing fluorinated benzyl group: synthesis, characterization, crystal structure, computational studies, and inhibitory properties against some metabolic enzymes. Appl Organomet Chem 35:6312. https://doi.org/10.1002/aoc.6312
Dvir H, Wong DM, Harel M et al (2002) 3D structure of Torpedo californica acetylcholinesterase complexed with huprine X at 2.1 Å resolution: kinetic and molecular dynamic correlates. Biochemistry 41:2970–2981. https://doi.org/10.1021/bi011652i
Nicolet Y, Lockridge O, Masson P et al (2003) Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J Biol Chem 278:41141–41147. https://doi.org/10.1074/jbc.M210241200
Auiewiriyanukul W, Saburi W, Kato K et al (2018) Function and structure of GH13_31 α-glucosidase with high α-(1→4)-glucosidic linkage specificity and transglucosylation activity. FEBS Lett 592:2268–2281. https://doi.org/10.1002/1873-3468.13126
Trott O, Olson AJ (2010) AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Fioravanti R, Desideri N, Carta A et al (2017) Inhibitors of Yellow Fever Virus replication based on 1,3,5-triphenyl-4,5-dihydropyrazole scaffold: design, synthesis and antiviral evaluation. Eur J Med Chem 141:15–25. https://doi.org/10.1016/j.ejmech.2017.09.060
Gul HI, Mete E, Taslimi P et al (2017) Synthesis, carbonic anhydrase I and II inhibition studies of the 1,3,5-trisubstituted-pyrazolines. J Enzyme Inhib Med Chem 32:189–192. https://doi.org/10.1080/14756366.2016.1244533
Abdel-Aziz AAM, El-Azab AS, Bua S et al (2019) Design, synthesis, and carbonic anhydrase inhibition activity of benzenesulfonamide-linked novel pyrazoline derivatives. Bioorg Chem 87:425–431. https://doi.org/10.1016/j.bioorg.2019.03.052
Donohoe TJ (2000) Oxidation and reduction in organic synthesis. Oxford University Press
Kısa D, Kaya Z, İmamoğlu R et al (2022) Assessment of antimicrobial and enzymes inhibition effects of Allium kastambulense with in silico studies: analysis of its phenolic compounds and flavonoid contents. Arab J Chem 15:103810. https://doi.org/10.1016/j.arabjc.2022.103810
Erdogan MK, Gundogdu R, Yapar Y et al (2022) The evaluation of anticancer, antioxidant, antidiabetic and anticholinergic potentials of endemic Rhabdosciadium microcalycinum supported by molecular docking study. ChemistrySelect 7:202200400. https://doi.org/10.1002/slct.202200400
Yakan H, Koçyiğit ÜM, Muğlu H et al (2022) Potential thiosemicarbazone-based enzyme inhibitors: assessment of antiproliferative activity, metabolic enzyme inhibition properties, and molecular docking calculations. J Biochem Mol Toxicol 36:23018. https://doi.org/10.1002/jbt.23018
Mohammadi-Khanaposhtani M, Nori M, Valizadeh Y et al (2022) New 4-phenylpiperazine-carbodithioate-N-phenylacetamide hybrids: synthesis, in vitro and in silico evaluations against cholinesterase and α-glucosidase enzymes. Arch Pharm (Weinheim) 355:2100313. https://doi.org/10.1002/ardp.202100313
Gülçin İ, Bingöl Z, Taslimi P et al (2022) Polyphenol contents, potential antioxidant, anticholinergic and antidiabetic properties of mountain mint (Cyclotrichium leucotrichum). Chem Biodivers 19:202100775. https://doi.org/10.1002/cbdv.202100775
Huseynova M, Farzaliyev V, Medjidov A et al (2022) Synthesis, biological and theoretical properties of crystal zinc complex with thiosemicarbazone of glyoxylic acid. J Mol Struct 1248:131470. https://doi.org/10.1016/j.molstruc.2021.131470
Huseynova A, Kaya R, Taslimi P et al (2022) Design, synthesis, characterization, biological evaluation, and molecular docking studies of novel 1,2-aminopropanthiols substituted derivatives as selective carbonic anhydrase, acetylcholinesterase and α-glycosidase enzymes inhibitors. J Biomol Struct Dyn 40:236–248. https://doi.org/10.1080/07391102.2020.1811772
Yiğit M, Celepci DB, Taslimi P et al (2022) Selenourea and thiourea derivatives of chiral and achiral enetetramines: synthesis, characterization and enzyme inhibitory properties. Bioorg Chem 120:105566. https://doi.org/10.1016/j.bioorg.2021.105566
Frisch MJ, Trucks GW, Schlegel HB et al (2010) Gaussian 09, Revision C. 01. Gaussian Inc., Wallingford, CT
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Legault CY (2009) CYLview, 1.0b. Univ. Sherbrooke, Queb́ec
Dennington R, Keith T, Millam J (2009) GaussView 5.0. Semichem Inc., Shawnee Mission, KS
Doğan ŞD, Çetinkaya Y, Buran S et al (2020) Chemoselective synthesis, X-ray characterization and DFT studies of new organic single crystal: S-(2-aminophenyl) cyclohexylcarbamothioate. J Mol Struct 1204:127499. https://doi.org/10.1016/j.molstruc.2019.127499
Doğan ŞD, Gündüz MG, Uğur SB et al (2021) Copper-oxone promoted oxidative C−H functionalization: synthesis of 2-aminobenzothiazoles and evaluation of their antimicrobial activities. ChemistrySelect 6:4382–4389. https://doi.org/10.1002/slct.202100485
Çetinkaya Y, Maraş A, Göksu S (2021) Insight into the intramolecular interactions of trans-2-azidocycloalk-3-en-1-ols and trans-2-azidocycloalk-3-en-1-yl acetates: a theoretical study. Tetrahedron 92:132272. https://doi.org/10.1016/j.tet.2021.132272
Demir E, Sari O, Çetinkaya Y et al (2020) One-pot synthesis of oxazolidinones and five-membered cyclic carbonates from epoxides and chlorosulfonyl isocyanate: theoretical evidence for an asynchronous concerted pathway. Beilstein J Org Chem 16:1805–1819. https://doi.org/10.3762/BJOC.16.148
Çetinkaya Y, Balci M (2014) Selective synthesis of N-substituted pyrrolo[1,2-a]pyrazin-1(2H)-one derivatives via alkyne cyclization. Tetrahedron Lett 55:6698–6702. https://doi.org/10.1016/j.tetlet.2014.10.044
Baskın D, Çetinkaya Y, Balci M (2018) Synthesis of dipyrrolo-diazepine derivatives via intramolecular alkyne cyclization. Tetrahedron 74:4062–4070. https://doi.org/10.1016/j.tet.2018.06.013
Glendening ED, Landis CR, Weinhold F (2013) NBO 6.0: natural bond orbital analysis program. J Comput Chem 34:1429–1437. https://doi.org/10.1002/jcc.23266
Weinhold F, Landis CR, Glendening ED (2016) What is NBO analysis and how is it useful? Int Rev Phys Chem 35:399–440. https://doi.org/10.1080/0144235X.2016.1192262
Gümüş HP, Tamer Ö, Avci D, Atalay Y (2014) Quantum chemical calculations on the geometrical, conformational, spectroscopic and nonlinear optical parameters of 5-(2-chloroethyl)-2,4- dichloro-6-methylpyrimidine. Spectrochim Acta - Part A Mol Biomol Spectrosc 129:219–226. https://doi.org/10.1016/j.saa.2014.03.031
Hiremath SM, Suvitha A, Patil NR et al (2018) Molecular structure, vibrational spectra, NMR, UV, NBO, NLO, HOMO-LUMO and molecular docking of 2-(4, 6-dimethyl-1-benzofuran-3-yl) acetic acid (2DBAA): experimental and theoretical approach. J Mol Struct 1171:362–374. https://doi.org/10.1016/j.molstruc.2018.05.109
Han Mİ, Dengiz C, Doğan ŞD et al (2022) Isoquinolinedione-urea hybrids: synthesis, antibacterial evaluation, drug-likeness, molecular docking and DFT studies. J Mol Struct 1252:132007. https://doi.org/10.1016/j.molstruc.2021.132007
Koopmans T (1934) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1:104–113. https://doi.org/10.1016/S0031-8914(34)90011-2
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Pearson RG (1986) Absolute electronegativity and hardness correlated with molecular orbital theory. Proc Natl Acad Sci 83:8440–8441. https://doi.org/10.1073/pnas.83.22.8440
Chattaraj PK, Sarkar U, Roy DR (2006) Electrophilicity index. Chem Rev 106:2065–2091. https://doi.org/10.1021/cr040109f
Murray JS, Politzer P (2011) The electrostatic potential: an overview. Wiley Interdiscip Rev Comput Mol Sci 1:153–163. https://doi.org/10.1002/wcms.19
Acknowledgements
The authors are grateful to Ataturk University for the financial support (BAP Projects 2016/142 and 2021/9687) of this work. Authors also thank for computer time provided by TUBITAK-ULAKBIM, High Performance and Grid Computing Center.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The authors reported there is no funding associated with the work featured in this article.
Author information
Authors and Affiliations
Contributions
YÇ, PT and AM designed and supervised this work and reviewed the manuscript. TA and AM synthesized the compounds and confirmed their structures. PT performed the biological activities of the compounds. YÇ was responsible for the computational studies, and carried out the molecular docking studies. YÇ, PT, and AM wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Artunç, T., Çetinkaya, Y., Taslimi, P. et al. Investigation of cholinesterase and α-glucosidase enzyme activities, and molecular docking and dft studies for 1,2-disubstituted cyclopentane derivatives with phenyl and benzyl units. Mol Divers (2024). https://doi.org/10.1007/s11030-024-10911-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-024-10911-y