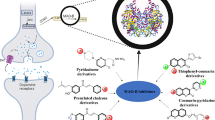
Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease affecting mental ability and neurocognitive functions. Cholinesterase enzymes affect concentration of acetylcholine in the brain, leading to dementia. Thus, there is an urgent need to develop novel dual cholinesterase inhibitors as possible anti-AD drugs. Herein, we have designed and synthesized a novel series of 9H-carbazole-4H-chromenes 4(a-l) through a one-pot three-component reaction of salicylaldehydes (1), hydroxycarbazole (2) and N-methyl-1-(methylthio)-2-nitroethenamine (3) using triethylamine as a catalyst in ethanol. Synthetic transformation involves the formation of two C–C bonds and one C–O bond in a single step to obtain desired analogs. The rapid one-pot reaction does not require chromatographic purification, proceeds under mild conditions, and exhibits good tolerance toward various functional groups with high synthetic yields. Synthesized compounds were screened for cytotoxicity using MTT assay in BV-2 microglial cells. These compounds were then in-vitro screened against acetylcholinesterase (AChE) and butyrylcholinestrase (BuChE) enzymes. Most of these ligands have shown dual cholinesterase inhibitory activity compared to the standard drug. In-vitro results showed that the compounds 4a and 4d have promising anticholinesterase response against both cholinesterase enzymes (4a, AChE IC50: 5.76 µM, BuChE IC50: 48.98 µM; 4d, AChE IC50: 3.58 µM, BuChE IC50: 42.73 µM). In-vitro results were validated by molecular docking and dynamic simulation at 100 ns. Molecular docking and molecular dynamics simulation study strongly supported structural features present in these analogs. Together, these analogs could be exploited to develop dual anti-cholinesterase candidates to treat AD in combination with other drugs.
Graphical abstract














Similar content being viewed by others
References
Patel DV, Patel NR, Kanhed AM, Patel SP, Sinha A, Kansara DD, Mecwan AR, Patel SB, Upadhyay PN, Patel KB, Shah DB, Prajapati NK, Murumkar PR, Patel KV, Yadav MR (2019) Multitarget directed triazinoindole derivatives as anti-Alzheimer agents. ACS Chem Neurosci 10:3635–3661
Patel DV, Patel NR, Kanhed AM, Teli DM, Patel KB, Gandhi PM, Patel SP, Chaudhary BN, Shah DB, Prajapati NK, Patel KV, Yadav MR (2020) Further studies on triazinoindoles as potential novel multitarget-directed anti-Alzheimer’s agents. ACS Chem Neurosci 11:3557–3574
Zeisel J, Bennett K, Fleming R (2020) World Alzheimer report: design, dignity, dementia: dementia-related design and the built environment. Alzheimer Disease International, London
Kasim SM, Dabbagh B, Mustafa YF (2022) A review on the biological potentials of carbazole and its derived products. Eurasian Chem Commun 4:495–512
Lokwani DK, Chavan SR, Ugale VG, Kendre PN, Jain SP (2024) Recent updates in chemistry of Alzheimer’s: synthetic molecules. Alzheimer’s disease and advanced drug delivery strategies. Academic Press, pp 33–46
Gutti G, Leifeld J, Kakarla R, Bajad NG, Ganeshpurkar A, Kumar A, Krishnamurthy S, Klein-Schmidt C, Tapken D, Hollmann M, Singh SK (2023) Discovery of triazole-bridged aryl adamantane analogs as an intriguing class of multifunctional agents for treatment of Alzheimer’s disease. Eur J Med Chem 259:115670
Varma M, Ugale V, Shaukat J, Hollmann M, Shete P, Shravage B, Tayade S, Kumbhar A, Butcher R, Jani V, Sonavane U (2023) Novel alkyl-substituted 4-methoxy benzaldehyde thiosemicarbazones: multi-target directed ligands for the treatment of Alzheimer’s disease. Eur J Pharmacol 957:176028
Cheung J, Gary EN, Shiomi K, Rosenberry TL (2013) Structures of human acetylcholinesterase bound to dihydrotanshinone I and territrem B show peripheral site flexibility. ACS Med Chem Lett 4:1091–1096
Li Q, Yang H, Chen Y, Sun H (2017) Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur J Med Chem 132:294–309
Nachon F, Carletti E, Ronco C, Trovaslet M, Nicolet Y, Jean L, Renard PY (2013) Crystal structures of human cholinesterases in complex with huprine W and tacrine: elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem J 453:393–399
Koca M, Güller U, Güller P, Dağalan Z, Nişancı B (2022) Design and synthesis of novel dual cholinesterase inhibitors: in-vitro inhibition studies supported with molecular docking. Chem Biodivers 19:e202200015
Patel KB, Patel DV, Patel NR, Kanhed AM, Teli DM, Gandhi B, Shah BS, Chaudhary BN, Prajapati NK, Patel KV, Yadav MR (2022) Carbazole-based semicarbazones and hydrazones as multifunctional anti-Alzheimer agents. J Biomol Struct Dyn 40:10278–10299
Abdulaziz NT, Al-bazzaz FY, Mustafa YF (2023) Natural products for attenuating Alzheimer’s disease: a narrative review. Eurasian Chem Commun 5:358–370
Kareem RT, Abedinifar F, Mahmood EA, Ebadi AG, Rajabi F, Vessally E (2021) The recent development of donepezil structure-based hybrids as potential multifunctional anti-Alzheimer’s agents: highlights from 2010 to 2020. RSC Adv 11:30781–30797
Reddy PN, Padmaja P, Reddy BR, Jadav SS (2017) Synthesis, in-vitro antiproliferative activity, antioxidant activity and molecular modeling studies of new carbazole Mannich bases. Med Chem Res 26:2243–2259
Padmaja P, Reddy PN, Reddy BVS, Misra S, Dhevendar K, Jadav SS (2017) Synthesis, molecular docking, in-vitro antiproliferative and antioxidant activity of novel pyrrolidinyl-carbazole derivatives. Curr Org Synth 14:1172–1179
Raj V, Lee J (2020) 2H/4H-Chromenes-A versatile biologically attractive Scaffold. Front Chem 8:623
Fernández-Bachiller MI, Pérez C, Monjas L, Rademann J, Rodríguez-Franco MI (2012) New Tacrine–4-oxo-4H-chromene hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with cholinergic, antioxidant, and β-amyloid-reducing properties. J Med Chem 55:1303–1317
Padmaja P, Reddy PN (2015) Hydroxycarbazoles as starting materials in organic syntheses. Curr Org Synth 12:3–19
Reddy PN, Padmaja P (2015) Applications of aminocarbazoles in heterocyclic synthesis. Arkivoc (i) 2015:244–268
Malathi V, Reddy PN, Padmaja P (2021) Novel one-pot synthesis of pyranocarbazolederivativesvia an isocyanide-based three-component reaction. Lett Org Chem 18:721–726
Padmaja P, Anireddy JS, Reddy PN (2018) Synthesis and antiproliferative activity of novel pyranocarbazoles. Chem Heterocycl Compd 54:812–818
Padmaja P, Reddy PN, Reddy BR (2001) An efficient synthesis of new fused oxazinocarbazoles. Lett Org Chem 15:653–658
Reddy PN, Padmaja P (2018) Rapid access of new pyranocarbazole derivatives under microwave irradiation. CurrMicrow Chem 5:104–110
Padmaja P, Reddy PN (2017) Microwave-promoted one-pot three-component synthesis of 1-amidoalkyl-2-carbazolol derivatives. Lett Org Chem 14:115–119
Padmaja P, Reddy BVS, Jain N, Mutheneni SR, Bollepelli P, Polepalli S, Rambabu G, Reddy PN (2016) Synthesis, molecular docking and in vitro antiproliferative activity of novel pyrano[3,2-c]carbazole derivatives. New J Chem 40:8305–8315
Reddy PN, Padmaja P, Reddy BR, Rambabu G, Kumar MP (2016) Synthesis, molecular docking, antiproliferative and antimicrobial activity of novel pyrano[3,2-c]carbazole derivatives. Med Chem Res 25:2093–2103
Padmaja P, Rao GK, Indrasena A, Reddy BVS, Patel N, Shaik AB, Reddy N, Dubey PK, Bhadra MP (2015) Synthesis and biological evaluation of novel pyrano[3,2-c]carbazole derivatives as anti-tumor agents inducing apoptosis via tubulin polymerization inhibition. Org Biomol Chem 13:1404–1414
Muralimohan G, Padmaja P, Aravind S, Reddy PN (2021) An efficient one-pot synthesis of indolyl-4H-chromene derivatives. Chem Heterocycl Compd 57:1176–1180
Nagaraju P, Padmaja P, Reddy PN (2019) Microwave-assisted one-pot synthesis of 7-dimethylamino-4-aryl-2-methylamino-3-nitro-4H-chromenes. Lett Org Chem 16:468–473
Reddy PN, Sharon N, Padmaja P, Ugale VG, Lokwani PD, Jain S, Pragati P, Anjali K (2023) Molecular hybridization-based design, PASE three-component synthesis, antiproliferative activity and molecular modelling studies of N, N-dimethylaminophenyl substituted 5H-chromeno[2,3-b]pyridine analogs. J Mol Str 1286:135589
Malathi V, Sharon N, Padmaja P, Lokwani D, Khadse S, Chaudhari P, Shirkhedkar AA, Reddy PN, Ugale VG (2023) Design, synthesis, and pharmacological evaluation of [1,3]dioxolo-chromeno[2,3-b] pyridines as anti-seizure agents. Mol Divers 27:1809–1827
Ugale V, Deshmukh R, Lokwani D, Reddy PN, Khadse S, Chaudhari P, Kulkarni PP (2023) GluN2B subunit selective N-methyl-D-aspartate receptor ligands: democratizing recent progress to assist the development of novel neurotherapeutics. Mol Divers. https://doi.org/10.1007/s11030-023-10656-0
Ugale VG, Bari SB, Khadse SC, Reddy PN, Bonde CG, Chaudhari P (2020) Exploring quinazolinones as anticonvulsants by molecular fragmentation approach: structural optimization, synthesis, and pharmacological evaluation studies. ChemistrySelect 5:2902–2912
Lee DS, Lee M, Sung SH, Jeong GS (2016) Involvement of heme oxygenase-1 induction in the cytoprotective and neuroinflammatory activities of Siegesbeckia Pubescens isolated from 5, 3′-dihydroxy-3, 7, 4′-trimethoxyflavone in HT22 cells and BV2 cells. IntImmunopharmaco 40:65–72
Vyas NA, Singh SB, Kumbhar AS, Ranade DS, Walke GR, Kulkarni PP, Jani V, Sonavane UB, Joshi RR, Rapole S (2018) Acetylcholinesterase and Aβ aggregation inhibition by heterometallic ruthenium (II)-platinum (II) polypyridyl complexes. Inorg Chem 57:7524–7535
Schrödinger release 2023–4: glide (2023). Schrödinger, LLC, New York.
Patil VR, Dhote AM, Patil R, Amnerkar ND, Lokwani DK, Ugale VG, CharbeNB FSD, Chaudhari P, Shah SK, Mehta CH, Nayak UY, Khadse SC (2023) Identification of structural scaffold from interbioscreen (IBS) database to inhibit 3CLpro, PLpro, and RdRp of SARS-CoV-2 using molecular docking and dynamic simulation studies. J BiomolStructDyn 22:13168–13179
Račková L, Csekes E (2022) Redox aspects of cytotoxicity and anti-neuroinflammatory profile of chloroquine and hydroxychloroquine in serum-starved BV-2 microglia. Toxicol Appl Pharmacol 447:116084
Rapaka D, Bitra VR, Challa SR, Adiukwu PC (2021) Potentiation of microglial endocannabinoid signaling alleviates neuroinflammation in Alzheimer’s disease. Neuropeptides 90:102196
Qin Q, Teng Z, Liu C, Li Q, Yin Y, Tang Y (2021) TREM2, microglia, and Alzheimer’s disease. Mech Ageing Dev 195:111438
Acknowledgements
VGU acknowledges the financial support from Department of Science and Technology (DST)-Science and Engineering Research Board (SERB), New Delhi, India (Ref. no. TAR/2021/000140). VGU is thankful to the Principal, R.C. Patel Institute of Pharmaceutical Education and Research, Shirpur, Maharashtra (India) for providing necessary facilities.PNR acknowledges GITAM (Deemed to be University) for research facilities. PP acknowledges the financial support of the Department of Science and Technology (DST) through the grant WOS-A (No.SR/WOS-A/CS-2/2019). PPK acknowledges the support from DST, India [Ref. no. CRG/2022/004365] and Agharkar Research Institute, Pune for providing research facilities. PS acknowledges the financial support from University Grants Commission, New Delhi, India through senior research fellowship. Authors thank the Director, Agharkar Research Institute, Pune for the support.
Author information
Authors and Affiliations
Contributions
NS: Methodology, Investigation; VGU: Conceptualization, Methodology, Investigation, Writing- Original draft preparation, Writing - Review & Editing; PP: Visualization, Data Curation; PNR: Methodology, Conceptualization, Interpretation, Resources, Review & Editing; PS: Investigation, Formal analysis; CS: Methodology, Investigation; DL: Methodology, Investigation; PPK: Supervision, Conceptualization, Methodology, Visualization, Writing - Review & Editing. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharon, N., Ugale, V.G., Padmaja, P. et al. Development of novel 9H-carbazole-4H-chromene hybrids as dual cholinesterase inhibitors for the treatment of Alzheimer’s disease. Mol Divers (2024). https://doi.org/10.1007/s11030-024-10859-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11030-024-10859-z