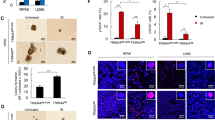
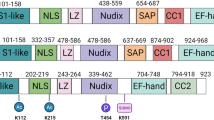
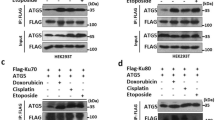
Abstract
DNA damage response (DDR) and autophagy are concerned with maintaining cellular homeostasis and dysregulation of these two pathways lead to pathologic conditions including tumorigenesis. Autophagy is activated as a protective mechanism during DDR which is indicative of their functional cooperativity but the molecular mechanism leading to the convergence of these two pathways during genotoxic stress remains elusive. In this study, through in silico analysis, we have shown an interaction between the Mediator of DNA damage checkpoint 1 (MDC1), an important DDR-associated protein, and Beclin-1, an autophagy inducer. MDC1 is an adaptor or scaffold protein known to regulate DDR, apoptosis, and cell cycle progression. While, Beclin-1 is involved in autophagosome nucleation and exhibits affinity for binding to Fork-head-associated domain (FHA) containing proteins. The FHA domain is commonly conserved in DDR-related proteins including MDC1. Through molecular docking, we have predicted the modeled complex between the MDC1 FHA domain and the Beclin-1 Coiled coil domain (CCD). The docking complex was modeled using ClusPro2.0, based on the crystal structure for the dimerized MDC1 FHA domain and Beclin-1 CCD. The complex stability and binding affinities were assessed using a Ramachandran plot, MD simulation, MM/GBSA, and PRODIGY webserver. Finally, the hot-spot residues at the interface were determined using computational alanine scanning by the DrugScorePPI webserver. Our analysis unveils significant interaction between MDC1 and Beclin-1, involving hydrogen bonds, non-bonded contacts, and salt bridges and indicates MDC1 possibly recruits Beclin-1 to the DSBs, as a consequence of which Beclin-1 is able to modulate DDR.
Graphical abstract








Similar content being viewed by others
References
Ruff SE, Logan SK, Garabedian MJ, Huang TT (2020) Roles for MDC1 in cancer development and treatment. DNA Repair (Amst). https://doi.org/10.1016/j.dnarep.2020.102948
Czarny P, Pawlowska E, Bialkowska-Warzecha J, Kaarniranta K, Blasiak J (2015) Autophagy in DNA damage response. Int J Mol Sci 16(2):2641–2662. https://doi.org/10.3390/ijms16022641
Petrini JHJ, Stracker TH (2003) The cellular response to DNA double-strand breaks: defining the sensors and mediators. Trends Cell Biol 13(9):458–462. https://doi.org/10.1016/S0962-8924(03)00170-3
Rassool FV (2003) DNA double strand breaks (DSB) and non-homologous end joining (NHEJ) pathways in human leukemia. Cancer Lett 193:1–9
So S, Davis AJ, Chen DJ (2009) Autophosphorylation at serine 1981 stabilizes ATM at DNA damage sites. J Cell Biol 187(7):977–990. https://doi.org/10.1083/jcb.200906064
Jungmichel S, Stucki M (2010) MDC1: the art of keeping things in focus. Chromosoma 119(4):337–349. https://doi.org/10.1007/s00412-010-0266-9
Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ (2001) ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem 276(45):42462–42467. https://doi.org/10.1074/jbc.C100466200
Jungmichel S, Clapperton JA, Lloyd J et al (2012) The molecular basis of ATM-dependent dimerization of the Mdc1 DNA damage checkpoint mediator. Nucleic Acids Res 40(9):3913–3928. https://doi.org/10.1093/nar/gkr1300
Coster G, Goldberg M (2010) The cellular response to dna damage: a focus on MDC1 and its interacting proteins. Nucleus. https://doi.org/10.4161/nucl.11176
Lou Z, Minter-Dykhouse K, Franco S et al (2006) MDC1 maintains genomic stability by participating in the amplification of ATM-dependent DNA damage signals. Mol Cell 21(2):187–200. https://doi.org/10.1016/j.molcel.2005.11.025
Lou Z, Minter-Dykhouse K, Wu X, Chen J (2003) MDC1 is coupled to activated CHK2 in mammalian DNA damage response pathways. Nature 421(6926):957–961. https://doi.org/10.1038/nature01447
Zhang J, Ma Z, Treszezamsky A, Powell SN (2005) MDC1 interacts with Rad51 and facilitates homologous recombination. Nat Struct Mol Biol 12(10):902–909. https://doi.org/10.1038/nsmb991
Panier S, Durocher D (2009) Regulatory ubiquitylation in response to DNA double-strand breaks. DNA Repair (Amst) 8(4):436–443. https://doi.org/10.1016/j.dnarep.2009.01.013
Nowsheen S, Lou Z (2018) Calling RNF168 to action. Cell Stress 2(5):113–114. https://doi.org/10.15698/cst2018.05.135
Goldberg M, Stucki M, Falck J et al (2003) MDC1 is required for the intra-S-phase DNA damage checkpoint. Nature 421(6926):952–956. https://doi.org/10.1038/nature01445
Luo K, Yuan J, Chen J, Lou Z (2009) Topoisomerase IIα controls the decatenation checkpoint. Nat Cell Biol 11(2):204–210. https://doi.org/10.1038/ncb1828
Leimbacher PA, Jones SE, Shorrocks AMK et al (2019) MDC1 interacts with TOPBP1 to maintain chromosomal stability during mitosis. Mol Cell 74(3):571-583.e8. https://doi.org/10.1016/j.molcel.2019.02.014
Nakanishi M, Ozaki T, Yamamoto H et al (2007) NFBD1/MDC1 associates with p53 and regulates its function at the crossroad between cell survival and death in response to DNA damage. J Biol Chem 282(31):22993–23004. https://doi.org/10.1074/jbc.M611412200
Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P (2008) Regulation of macroautophagy by mTOR and Beclin 1 complexes. Biochimie 90(2):313–323. https://doi.org/10.1016/j.biochi.2007.08.014
Alexander A, Cai SL, Kim J et al (2012) Erratum: ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS (Proceedings of the National Academy of Sciences (2010) 107 (4153-4158) https://doi.org/10.1073/pnas.0913860107). Proc Natl Acad Sci USA 109(21):8352. https://doi.org/10.1073/pnas.1206201109
Liu EY, Xu N, O’Prey J et al (2015) Loss of autophagy causes a synthetic lethal deficiency in DNA repair. Proc Natl Acad Sci USA 112(3):773–778. https://doi.org/10.1073/pnas.1409563112
Yue Z, Jin S, Yang C, Levine AJ, Heintz N (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA 100(25):15077–15082. https://doi.org/10.1073/pnas.2436255100
Qu X, Yu J, Bhagat G et al (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 112(12):1809–1820. https://doi.org/10.1172/JCI20039
Chavez-Dominguez R, Perez-Medina M, Lopez-Gonzalez JS, Galicia-Velasco M, Aguilar-Cazares D (2020) The double-edge sword of autophagy in cancer: from tumor suppression to pro-tumor activity. Front Oncol 10(October):1–19. https://doi.org/10.3389/fonc.2020.578418
Xu F, Fang Y, Yan L et al (2017) Nuclear localization of Beclin 1 promotes radiation-induced DNA damage repair independent of autophagy. Sci Rep. https://doi.org/10.1038/srep45385
Guo Q, Wang S, Zhang S et al (2020) ATM - CHK 2-Beclin 1 axis promotes autophagy to maintain ROS homeostasis under oxidative stress. EMBO J 39(10):1–17. https://doi.org/10.15252/embj.2019103111
Chang YC, Peng YX, Yu BH et al (2021) VCP maintains nuclear size by regulating the DNA damage-associated MDC1–p53–autophagy axis in Drosophila. Nat Commun 12(1):1–17. https://doi.org/10.1038/s41467-021-24556-0
Rose PW, Beran B, Bi C et al (2011) The RCSB Protein Data Bank: redesigned web site and web services. Nucleic Acids Res 39(SUPPL. 1):392–401. https://doi.org/10.1093/nar/gkq1021
Pettersen EF, Goddard TD, Huang CC et al (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612. https://doi.org/10.1002/jcc.20084
Kozakov D, Hall DR, Xia B et al (2017) The ClusPro web server for protein-protein docking. Nat Protoc 12(2):255–278. https://doi.org/10.1038/nprot.2016.169
Laskowski RA, Jabłońska J, Pravda L, Vařeková RS, Thornton JM (2018) PDBsum: structural summaries of PDB entries. Protein Sci 27(1):129–134. https://doi.org/10.1002/pro.3289
Unit M, Street G (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. https://doi.org/10.1107/S0021889892009944
Najibi SM, Maadooliat M, Zhou L, Huang JZ, Gao X (2017) Protein structure classification and loop modeling using multiple ramachandran distributions. Comput Struct Biotechnol J 15:243–254. https://doi.org/10.1016/j.csbj.2017.01.011
Xue LC, Rodrigues JP, Kastritis PL, Bonvin AM, Vangone A (2016) PRODIGY: a web server for predicting the binding affinity of protein-protein complexes. Bioinformatics 32(23):3676–3678. https://doi.org/10.1093/bioinformatics/btw514
Vangone A, Bonvin A (2017) PRODIGY: a contact-based predictor of binding affinity in protein-protein complexes. Bio-Protoc. https://doi.org/10.21769/BIOPROTOC.2124
Massova I, Kollman PA (1999) Computational alanine scanning to probe protein−protein interactions: a novel approach to evaluate binding free energies. J Am Chem Soc 3:1–11
Krüger DM, Gohlke H (2010) DrugScorePPI webserver: fast and accurate in silico alanine scanning for scoring protein-protein interactions. Nucleic Acids Res 38(SUPPL. 2):480–486. https://doi.org/10.1093/nar/gkq471
Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJC (2005) GROMACS: fast, flexible, and free. J Comput Chem 26(16):1701–1718. https://doi.org/10.1002/jcc.20291
Lindahl E, Bjelkmar P, Larsson P, Cuendet MA, Hess B (2010) Implementation of the charmm force field in GROMACS: analysis of protein stability effects from correction maps, virtual interaction sites, and water models. J Chem Theory Comput 6(2):459–466. https://doi.org/10.1021/ct900549r
Peng X, Wang J, Peng W, Wu FX, Pan Y (2017) Protein-protein interactions: detection, reliability assessment and applications. Brief Bioinform 18(5):798–819. https://doi.org/10.1093/bib/bbw066
Chen F, Sun H, Wang J et al (2018) Assessing the performance of MM/PBSA and MM/GBSA methods. 8. Predicting binding free energies and poses of protein-RNA complexes. RNA 24(9):1183–1194. https://doi.org/10.1261/rna.065896.118
Weng G, Wang E, Wang Z et al (2019) HawkDock: a web server to predict and analyze the protein-protein complex based on computational docking and MM/GBSA. Nucleic Acids Res 47(W1):W322–W330. https://doi.org/10.1093/nar/gkz397
Stitou M, Toufik H, Bouachrine M, Lamchouri F (2021) Quantitative structure–activity relationships analysis, homology modeling, docking and molecular dynamics studies of triterpenoid saponins as Kirsten rat sarcoma inhibitors. J Biomol Struct Dyn 39(1):152–170. https://doi.org/10.1080/07391102.2019.1707122
Maréchal A, Zou L (2013) DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol 5(9):1–17. https://doi.org/10.1101/cshperspect.a012716
Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP (2005) MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 123(7):1213–1226. https://doi.org/10.1016/j.cell.2005.09.038
Qiu XLZ, Zuo ZWW, Liu ZGC (2017) NFBD1 / MDC1 participates in the regulation of proliferation and apoptosis in human laryngeal squamous cell carcinoma. Clin Transl Oncol. https://doi.org/10.1007/s12094-017-1748-5
Singh N, Bhakuni R, Chhabria D, Kirubakaran S (2020) MDC1 depletion promotes cisplatin induced cell death in cervical cancer cells. BMC Res Notes 13(1):1–9. https://doi.org/10.1186/s13104-020-04996-5
Ruff SE, Logan SK, Garabedian MJ, Huang TT (2020) Roles for MDC1 in cancer development and treatment. DNA Repair (Amst) 95(August):102948. https://doi.org/10.1016/j.dnarep.2020.102948
Dziadkowiec KN, Gasiorowska E, Nowak-Markwitz E, Jankowska A (2016) PARP inhibitors: review of mechanisms of action and BRCA1/2 mutation targeting. Prz Menopauzalny 15(4):215–219. https://doi.org/10.5114/pm.2016.65667
Shin DW (2020) Dual roles of autophagy and their potential drugs for improving cancer therapeutics. Biomol Ther 28(6):503–511. https://doi.org/10.4062/biomolther.2020.155
Lin JF, Lin YC, Tsai TF, Chen HE, Chou KY, Hwang TIS (2017) Cisplatin induces protective autophagy through activation of BECN1 in human bladder cancer cells. Drug Des Devel Ther 11:1517–1533. https://doi.org/10.2147/DDDT.S126464
Mathew R, Karp CM, Beaudoin B et al (2009) Autophagy suppresses tumorigenesis through elimination of p62. Cell 137(6):1062–1075. https://doi.org/10.1016/j.cell.2009.03.048
Cicchini M, Chakrabarti R, Kongara S et al (2014) Autophagy regulator BECN1 suppresses mammary tumorigenesis driven by WNT1 activation and following parity. Autophagy 10(11):2036–2052. https://doi.org/10.4161/auto.34398
Patel AN, Goyal S, Wu H, Schiff D, Moran MS, Haffty BG (2011) Mediator of DNA damage checkpoint protein 1 ( MDC1) expression as a prognostic marker for nodal recurrence in early-stage breast cancer patients treated with breast-conserving surgery and radiation therapy. Breast Cancer Res Treat 1:601–607. https://doi.org/10.1007/s10549-010-0960-6
Caracciolo D, Riillo C, Teresa M, Martino D, Tagliaferri P (2021) Repair as cancer’s Achilles ’ heel
Acknowledgements
The authors acknowledge the Indian Institute of Advanced Research Gandhinagar for a seed grant to carry out this work. We would like to thank Dr. Ashutosh Srivastava, Assistant Professor, Biological Engineering, Indian Institute of Technology-Gandhinagar (IITGN) for his valuable comments related to the structural analysis of proteins. We also acknowledge Lady Tata Memorial Trust (LTMT) for providing fellowship to Ms. Kavya Pandya. The authors are thankful to the Gujarat Council of Science and Technology (GUJCOST), Dept. of Science and Technology, Gujarat for providing the PARAM SHAVAK supercomputer facility at IAR.
Author information
Authors and Affiliations
Contributions
NS did the conceptualization of the study. NS and KP designed the study. KP performed the in silico experiments. NS and KP wrote the manuscript. Both the authors have contributed equally in reviewing and editing the manuscript and approve the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they do not have any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pandya, K., Singh, N. In silico study reveals unconventional interactions between MDC1 of DDR and Beclin-1 of autophagy. Mol Divers 27, 2789–2802 (2023). https://doi.org/10.1007/s11030-022-10579-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10579-2