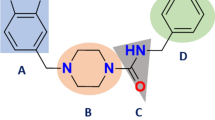
Abstract
In our continued efforts to find potential chemotherapeutics active against drug-resistant (DR) Mycobacterium tuberculosis (Mtb), causative agent of Tuberculosis (TB) and to curb the current burdensome treatment regimen, herein we describe the synthesis and biological evaluation of urea and thiourea variants of 5-phenyl-3-isoxazolecarboxylic acid methyl esters as promising anti-TB agent. Majority of the tested compounds displayed potent in vitro activity not only against drug-susceptible (DS) Mtb H37Rv but also against drug-resistant (DR) Mtb. Cell viability test against Vero cells deemed these compounds devoid of significant toxicity. 3,4-Dichlorophenyl derivative (MIC 0.25 µg/mL) and 4-chlorophenyl congener (MIC 1 µg/mL) among urea and thiourea libraries respectively exhibited optimum potency. Lead optimization resulted in the identification of 1,4-linked analogue of 3,4-dichlorophenyl urea derivative demonstrating improved selectivity. Further, in silico study complemented with previously proposed prodrug like attributes of isoxazole esters. Taken together, this molecular hybridization approach presents a new chemotype having potential to be translated into an alternate anti-Mtb agent.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent past has seen resurgence of tuberculosis (TB) drug research, ignited primarily by an urgent need to curtail TB epidemic as it continues to be the leading cause of death amongst infectious diseases globally, resulting in severe disruption in public health and socio-economic status. Increasing instances of drug resistance (DR), not only to existing drugs, but also to those in clinical pipeline pose a stiff challenge for drug development efforts, amidst concerning report from WHO stating years of progress being reversed by the ongoing coronavirus pandemic (COVID-19) [1, 2]. Apart from disrupting essential TB services and assess to TB diagnosis and healthcare system, co-infection of TB and COVID-19, i.e., COVID-TB is emerging as a major threat to global TB burden and associated mortality [3]. Drug susceptible (DS) TB alone necessitates multiple 1st line drugs (Isoniazid, Rifampicin, Ethambutol, Pyrazinamide) while resistance to these leads to DR-TB requiring a cocktail of not so effective and often toxic 2nd line anti-TB drugs for a longer duration. This predisposes the current treatment protocol to non-adherence amongst patients further exposing them to development of multi and extensively drug-resistant (MDR & XDR) strains. This emphasizes the need for effective drugs to combat ever hovering DR and to shorten and simplify TB treatment regimen so as to reduce associated adverse effects [4, 5]. In addition, the escalation in latent TB infection (LTBI) cases that was estimated to inhabit nearly one-quarter of world population in 2014 with a potential lifelong risk of reactivation in immune compromised population adversely impacts already unwieldy TB treatment protocol [6]. In this context, it has been proposed that, a shift from contemporary privileged anti-TB classes to entirely new scaffolds possibly acting on previously unknown targets could efficiently address this issue [7]. Therefore, continual feeding of research pipeline is imperative to accomplish WHO’s milestone of End TB Strategy of 90% reduction in annual TB death by 2030 [1]. Despite countless endeavours in anti-TB drug discovery in recent years, there continues to be scarcity of new drug approval with bedaquiline, delamanid and pretomanid being sole candidate to get regulatory nod during last decade while still undergoing additional clinical trials as combination therapy [8]. Apparently, anti-TB drug discovery research revolves around very limited privileged chemical classes and targets [9, 10]. Owing to the challenge in permeability posed by complex waxy nature of Mtb cell envelope, majority of these anti-TB chemotypes and targets are focused on extracellular pathway [11, 12].
On the contrary, a unique target that has attracted considerable attention in recent years is Mtb protein-tyrosine-phosphatase (Mptp), a virulence factor secreted by Mtb into host macrophages, compromising their immune response, thereby assisting in intra-macrophage survival and persistence of Mtb infection in host [13]. Many isoxazole acids are reported as potent inhibitors of MptpB exemplified by representative compound I depicted in Fig. 1 [14,15,16]. Evidently, these compounds have no effect on growth of Mtb, rather these synergistically enhance potency of first-line anti-TB drugs. On the flip side, target-based approaches have hardly produced any clinical candidates over last two decades. Rather, whole-cell screening approach has contributed immensely to anti-TB clinical pipeline [10]. One such scaffold yielded by this method is isoxazole esters, that are hypothesized to be prodrugs with pyrazinamide like action [17]. The fact that, these could act as ideal replacement for many nitro-containing potential mutagenic and genotoxic anti-TB scaffolds like nitrofuran, nitro-thiophene, nitroimidazole [18], owing to their bio-isosteric resemblance to them [19] provides additional advantage. Several isoxazole carboxylic acid alkyl ester derivatives like II depicted in Fig. 1 are reported in literature as potent inhibitor of not only drug-susceptible (DS) but also drug-resistant (DR) Mtb [19,20,21,22,23,24,25]. Apart from isoxazole acid and ester, the peculiar and versatile anti-TB attributes of isoxazole moiety as an anti-TB pharmacophore could be well judged from several representative compounds reported in literature [26,27,28].
Illustration of design rationale: green circle indicates part of structure altered from previously reported anti-TB compounds (II, IV) for designing target compounds. Predicted key interactions displayed by IV in active site of MmpL3 protein (PDB code: 6AJH) and similarity extrapolation for target compound
Similarly, urea and thiourea functionalities are widespread in literature but clinically less explored as anti-TB pharmacophores. Urea and thiourea containing compounds are appealing to medicinal chemists because of their key drug-target interactions and crucial drug-like properties [29]. Presence of both H-bond acceptor/donor and owing to their linear shape, these motifs aid in bioavailability and hydrophilicity [30].
Indeed, many urea and thiourea derivatives have been reported as potential anti-TB agents in literature portraying diverse mechanism of action [29, 31,32,33,34,35,36]. Amongst these, adamantyl ureas are emerging anti-TB chemotypes epitomised by compound III, as inhibitor of Mycobacterial membrane protein Large 3 (MmpL3), a protein required for transport of trehalose monomycolates (TMMs) across the cell membrane of Mtb for cell wall biosynthesis [37, 38]. However, utility of these inhibitors is impeded by poor solubility leading to low bioavailability and lack of selectivity, since these also inhibit human soluble epoxide hydrolase (hsEH) [39]. These issues were successfully addressed by the introduction of polar groups and heteroaromatics to adamantyl urea scaffold as in IV (Fig. 1) [40, 41]. In a similar pursuit, the present study aspires to introduce polar isoxazole ester motif to urea and thiourea core in search of a promising anti-TB agent. i.e., by keeping the urea/thiourea motif (that are essential for key polar interaction with Asp-645, Tyr-646 of MmpL3) intact, polar salicylate moiety of previous anti-TB compound IV was replaced with 5-phenyl isoxazole-3-carboxylic acid methyl ester motif in the present study to find similarly improved physicochemical attribute as that of IV. The present work aims to find out whether the target compounds exhibit anti-TB potency while retaining acceptable physicochemical attribute with speculative or possible MmpL3 inhibition.
The fact that, favourable attributes of isoxazole motif like presumable dual mode of activity besides being an ideal replacement for rather toxic nitro-containing anti-TB agents, suited well with the rationale. On the other hand, linear or planar linkers significantly potentiated anti-TB activity of isoxazole esters that has been revealed through previous SAR studies and has also been validated in our earlier work [19]. In this context, urea/thiourea motif, being a linear pharmacophore besides possessing favourable physicochemical properties complemented with the rationale (Fig. 1) that seeks to find out whether scaffold hybridization of urea/thiourea and isoxazole ester moiety could yield a potent anti-TB compound.
Result and discussion
Chemistry
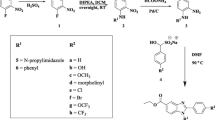
Synthesis of 40 new target compound was envisaged in an aim to identify optimum ureido/thioureido analogue of projected molecule as outlined in Scheme 1A. Key intermediate anilines were synthesized employing previously reported literature [19].
A–C Synthetic route to 5-phenyl-3-isoxazolecarboxylic acid methyl ester linked urea (4a–y), thiourea derivatives (5a–o) and optimized compounds (4ta–te). Reagents and conditions: (i) diethyl oxalate or dimethyl oxalate, t-BuONa, THF, 0 °C to rt., 2 h; (ii) a NH2OH.HCl, EtOH or MeOH, Reflux, 6 h; b. Na2S2O4, EtOH/MeOH:H2O (1:1), 65 °C, 1 h, 45–53% (3 steps); (iii) dry THF, rt, N2, 16–18 h, 40–88%; (iv) dry ACN, rt, N2, 16–18 h, 37–77%; (v) dry ACN, TEA, reflux, N2, 18–20 h, 54–56%; (vi) a NaNO2, NaN3, conc. HCl:H2O 1:1, 0 °C-rt, 2 h; b ethyl acetoacetate, TEA, DMF, rt, overnight, 60%; c. H2, Pd/C, Dry EtOAc, rt, 2 h, 78%; (vii) LiOH.H2O, THF: H2O (9:1), rt, 4 h, 92%
Briefly, substituted acetophenones 1a–c were treated with appropriate diesters of oxalic acid in presence of sodium tert-butoxide to furnish 2,4-diketoester 2a–d. Cyclization of resultant intermediate 2a–d with hydroxylamine hydrochloride led to the formation of nitro substituted isoxazole intermediate, that on subsequent reduction by sodium dithionite formed the desired aniline intermediate 3a–d. Key intermediate 5-(3-aminophenyl)isoxazole-3-methyl ester 3a was treated with various commercially available substituted phenyl isocyanates and benzyl isocyanates in dry tetrahydrofuran under inert condition at room temperature to provide urea derivatives 4a–x and 4y, respectively. Similarly, thiourea analogues 5a–m were obtained by treatment of commercially available substituted phenyl isothiocyanates with intermediate 3a using dry acetonitrile as solvent under inert atmosphere at room temp. Refluxing n-hexyl and cyclohexyl isothiocyanate with 3a in presence of base triethylamine in dry acetonitrile under inert condition led to the formation of aliphatic thiourea analogues 5n and 5o, respectively. Structural modification pertaining to variation in isoxazole counterpart of title molecule was accomplished by combining optimized ureido/thioureido portion with different congeners of 5-(3-aminophenyl)isoxazole-3-methyl ester 3a, i.e., ethyl ester analogue 3b, para-amino substituted regio-isomer 3c, methoxy-substituted derivative 3d. Coupling of 3,4-dichlorophenyl isocyanate with 3b, 3c, 3d using a similar procedure as has been used for synthesis of urea derivatives led to the formation of 4ta, 4tb and 4tc, respectively (Scheme 1A). A similar congener of 3a, i.e., triazole ester intermediate 7 was synthesized from 3-nitroaniline by tandem diazotization, followed by sequential replacement with azide, cyclization with ethyl acetoacetate and reduction. Condensation of 7 with 3,4-dichlorophenyl isocyanate using similar procedure as that of synthesis of urea derivative furnished 4td (Scheme 1B). Further, 4t was subjected to basic hydrolysis with lithium hydroxide to form 4te (Scheme 1C).
Anti-TB activity
The antimycobacterial activity of synthesized compounds was evaluated by broth microdilution method with resazurin as a metabolic indicator. The concentration of newly synthesized compounds was tested from 64 to 0.03 µg/mL along with Isoniazid (INH), Rifampicin (RIF), Streptomycin (STR) and Ethambutol (ETB) as reference standards. The preliminary screening indicated that synthesized compounds were selectively active towards Mtb H37Rv while showing no inhibition of non-tuberculous mycobacteria (NTM) i.e., M. abscessus ATCC 19977, M. fortuitum ATCC 6841 and M. chelonae ATCC 35752. However, weaker inhibition against M. fortuitum and M. chelonae was observed with thiourea analogues 5i, 5k, and 5m. Nevertheless, these showed better selectivity towards Mtb. Complete antimycobacterial screening results of urea derivatives 4a–y and thiourea analogues 5a–o are listed in Supplementary information (Table S1). To determine their selectivity, these compounds were also tested against a panel of bacteria i.e., E. coli ATCC 25922, S. aureus ATCC 29213, K. pneumoniae BAA 1705, A. baumannii BAA 1605 and P. aeruginosa ATCC 27853 (Table S2 in supplementary information). Screening results against bacterial pathogen panel demonstrated no activity by all compounds except 4n, 4q and 5l showing minimal inhibition (8–16 µg/mL) against S. aureus, hence indicating their specificity towards Mtb. Anti-TB activity of all evaluated compounds is listed in Table 1.
Out of 40 evaluated compounds, most of the compounds exhibited significant potency against Mtb H37Rv (MIC 0.25–2 µg/mL). Comparatively, urea derivatives 4a–y were found to be more potent than thiourea analogues 5a–o. Detailed scrutiny of urea derivatives indicated compounds having halogen substituents among both mono- and di-substituted compounds displayed potent anti-TB activity. In contrast, m-cyano, p-methoxy bearing substituents displayed poor to moderate potency. These results implied that halogen moiety (non-polar group) at m-/p- position and methoxy group at o- position were optimal for anti-TB potency. Methyl group was found to be well tolerated to enhance potency while polar substituents like cyano was detrimental for activity. Similarly, among disubstituted urea derivatives dichloro- bearing groups (4q, 4r, 4t) were found to display superior anti-TB potency. Surprisingly, compounds 4u, 4v, 4x were found to be weakly active or inactive despite having favourable non-polar substituents. Interestingly, all these compounds had either methyl or trifluoromethyl substitution. These groups are considered to have larger conformation, hence when present in proximity with similar larger groups (chloro- or methyl), they probably created steric influence that possibly accounted for their poor inhibitory activity. Apart from the aforementioned aryl substituted analogues, benzyl substituted urea derivative 4y was well tolerated displaying moderate anti-TB potency (MIC 4 µg/mL). Overall, compound 4t bearing 3,4-dichlorophenyl moiety was found to exhibit optimum potency amongst urea derivatives (MIC 0.25 µg/mL).
On the other hand, thiourea derivatives irrespective of having electronically divergent substituents, displayed almost similar anti-TB potency. Chloro- analogues were found to exhibit optimum potency irrespective of position among monosubstituted thiourea derivatives with p-chloro (5f) being the most active (MIC 1 µg/mL). Contrary to excellent potency of m-halogen urea derivatives, m-chloro (5c) and m-bromo (5d) bearing thiourea analogues showed moderate potency. Among disubstituted analogues, 2,4-difluoro derivative (5k) exhibited good activity (MIC 2 µg/mL) while compound 5m showed moderate potency owing to the presence of moderately tolerable m-chloro group which is consistent with trend observed with disubstituted urea derivatives. Similarly, compound 5l showed weak potency on basis of its bulky substitution (two trifluoromethyl groups) complementing to that of urea derivatives. Apart from these aryl derivatives, aliphatic group bearing thiourea analogue displayed excellent potency with both n-hexyl (5n) and cyclohexyl (5o) derivatives inhibiting Mtb (MIC 2 µg/mL). All these broad SAR observations are summarized in Fig. 4. Among urea derivatives 4t (MIC 0.25 µg/mL) bearing 3,4-dichlorophenyl substitution and from thiourea analogues 5f (MIC 1 µg/mL) having 4-chlorophenyl moiety, were found to be the potent compounds of the series.
Lipophilicity is governed by Clog P that controls membrane permeability—a critical factor for anti-TB potency [42]. In an attempt to validate correlation between lipophilicity and anti-TB activity, a plot between Clog P (Table S1 in supplementary information) and anti-TB potency of synthesized compounds was constructed (Fig. 2). A compound needs to be lipophilic enough to penetrate through unique nature of Mtb cell wall comprising mycolic arabinogalactan-peptidoglycan complex to exert its inhibitory action, while at same time limiting off-target impact. A generalized inference from the plot indicated that bulk of the compounds with higher Clog P value ended up with potent anti-TB activity. Most potent compounds of the series exhibiting anti-TB MIC of ≤ 1 µg/mL had Clog P value > 3.5. Conversely, weakly active compounds were found to have lower Clog P values. Interestingly, thiourea derivatives had lower Clog P than urea analogues, impact of which was reflected in their potency with thiourea derivatives being less potent as compared to urea analogues.
However, 4u, 4v and 5l were poorly active or inactive despite possessing Clog P value close to 5. This anomalous behaviour might be due to steric effect of the hindered functional groups (methyl, trifluoromethyl in close proximity to one another or halogen), thus leading to restricted target interaction. Aforementioned observation is similar to a previous work on prototype MmpL3 inhibitor indole-2-carboxamide anti-TB pharmacophores, wherein larger groups like methyl, methoxy, cyano produced comparatively reduced potency than smaller groups like chloro and fluoro [43, 44].
Structural modification of most potent analogue and its outcome
The optimum active compound 4t was subjected to optimization wherein variation was performed on 5-phenyl-3-isoxazolecarboxylic acid methyl ester fragment in an attempt to further potentiate the molecule and derive detailed structure activity relationship (SAR). In this context, methyl ester was modified by adding alkyl appendage and hydrolysed to evaluate its impact on potency. Similarly, regioisomer having different linker and replacement of isoxazole moiety was planned to understand compound alignment and importance of isoxazole moiety in eliciting anti-TB activity, respectively. Besides this, a potential blocking group was introduced in close proximity to ureido moiety to restrict exposure of ureido motif to study its necessity for interaction with target.
To accomplish the aforementioned, 4ta (ethyl ester analogue of 4t); 4tb (regio-isomer of 4t; having para ureido-linkage rather than meta) and 4tc (methoxy-substituted derivative) were synthesized. Further, isoxazole methyl ester of 4t was replaced by triazole ethyl ester moiety to obtain compound 4td, which was synthesized with an aim to investigate importance of isoxazole ester in imparting anti-TB property. To establish significance of ester motif, it was hydrolysed to corresponding carboxylic acid to obtain 4te. Figure 3 shows variations made, highlighting part of the molecule altered. All these compounds were screened against mycobacterial and bacterial pathogen panel that suggested these compounds were selective towards Mtb (Tables S1 and S2 in supplementary information). Both compounds 4ta and 4tb displayed four- and twofold decrease in anti-TB potency to that of 4t respectively suggesting neither introduction of alkyl appendage nor change in position of ureido linker is favourable for potency. On the other hand, both 4tc and 4td displayed significant reduction in potency with MIC 8 and 16 µg/mL, respectively. The observed moderate activity of 4td signified the essential nature of isoxazole ester for anti-TB activity. However, unlike our earlier work [19], wherein a similar alteration of isoxazole with triazole moiety resulted in complete loss of activity, in contrast to 4td, that retained moderate activity indicating innate activity of urea core towards Mtb. These facts validated the rationale of this design that introduction of urea core potentiated anti-TB activity of isoxazole ester motif. Similarly, the deterioration of potency for 4tc indicated that introduction of methoxy group adjacent to ureido moiety probably caused reduced interaction of ureido motif with target. This could further be justified from the fact that a similar modification in our earlier work resulted in producing most potent molecule wherein, the methoxy group possibly hindered metabolic susceptibility of adjacent amide linkage [25]. On the other hand, 4te was found to be poorly active towards Mtb, indicating importance of ester moiety in imparting lipophilicity required for permeability through cell wall of Mtb, that is consistent with previous literature [17]. These findings from structural modifications are summarized as SAR in Fig. 4.
Cell viability assay against Vero cells
Cytotoxicity evaluation of most potent compounds having MIC ≤ 1 µg/mL was carried out against Vero cells to verify whether the target compounds are active because of their toxicity. i.e., to verify whether the target compounds non-selectively kill both mammalian as well as mycobacterial cell. CC50 is the lowest concentration of compound that causes 50% cell death. Selectivity index (SI) is the ratio between the concentration of compound that is toxic to mammalian Vero cells (CC50) to the concentration that can kill the mycobacteria (MIC). From the results, it was observed that most of tested compounds displayed low cytotoxicity to Vero cells as inferred from favourable CC50 of > 10 µg/mL along with a high selectivity index (SI) of > 20. This indicated that, the target compounds are not cytotoxic at a conc. of 20 times MIC, suggesting a broad therapeutic index. This confirmed that the anti-TB activity of these compounds doesn’t stem from toxicity. The results are tabulated in Table 2.
However, 4d and 4w were found to have low selectivity index (SI) exhibiting CC50 < 10 µg/mL (SI < 20). Overall, the results suggested that among urea series, 4j was found to be most selective with CC50 > 100 µg/mL and SI > 200. The lone compound tested from thiourea series 5f showed CC50 > 50 µg/mL with SI > 50. Compounds obtained through structural variation i.e., 4ta and 4tb were also subjected to cytotoxicity analysis against Vero cells and were found to be highly selective as compared to 4t. While 4ta exhibited marginal improvement in selectivity; 4tb displayed significant increase in selectivity with CC50 > 40 µg/mL and SI > 80 indicating that linear nature of molecule incorporated by para substitution conferred better selectivity.
Determination of activity against clinical isolates of DR-Mtb
Non-toxic potent compounds (MIC ≤ 1 µg/mL) were further evaluated for their activity against clinical isolates of DR-Mtb strains. The results obtained are tabulated in Table 2. Most of the compounds tested exhibited moderate inhibitory activity against DR strains. Compounds were found to be highly active towards INH- and ETB-resistant strains while exhibiting moderate to poor inhibition of STR- and RIF-resistant strains in general while, exhibiting resistance towards STR-res strains in particular. Surprisingly, 4j (considering MIC 0.5 µg/mL and SI > 200) was revealed to be least active against resistant strains among all tested compounds. Interestingly, compound 4t and 5f, that happened to be the most potent compound of their respective series against susceptible Mtb strains, also inhibited all DR-Mtb strains, although not equipotently (MIC 0.5–4 µg/mL). Specifically, encouraging result was observed with 4t inhibiting both INH and STR-res strains (MIC 2 µg/mL) while displaying stronger inhibition against ETB and RIF-res strains (MIC 1 and 0.5 µg/mL, respectively). On the other hand, structurally modified analogues of 4t; compound 4ta and 4tb exhibited mixed result. 4ta was found to be highly active against all DR strains except STR-res strains wherein it showed moderate potency. On the contrary, 4tb displayed better activity towards all DR strains with MIC 1–4 µg/mL. Results of structural modification signified optimal nature of methyl ester for anti-TB potency while a linear shape is essential for better selectivity. The target compounds were found more potent towards sensitive strains (DS-Mtb) than resistant ones (DR-Mtb). Since this cannot be attributed to mutation considering these share probably different MOA that standard drugs taken as reference in this study; the possible reason could be attributed to enzymatic inactivation or efflux mechanism rather than mutation [45,46,47]. Previous studies pointed out metabolic susceptibility of esters leading to the formation of isoxazole acids that probably displayed pyrazinamide like mechanism of action [17, 21]. However, the present study attempted to keep metabolically labile ester while exploring the pharmacophoric attributes of urea and thiourea motif as an anti-TB scaffold. Taken together, the molecular hybridization strategy successfully delivered potent anti-TB lead against DS-Mtb while exhibiting marginally reduced potency against DR-Mtb. To sum up, based on better anti-TB potency against both susceptible and resistant strains and its non-toxic attribute; 4t was identified to be the most potent compound of the series.
Structure activity relationship (SAR) and mechanistic basis of anti-TB activity
SAR features (Fig. 4) of present study led to following considerations; (a) isoxazole esters 4t exhibited significant anti-TB potency as compared to its acid counterpart 4te that complemented with the prodrug like action; (b) being a linear moiety, urea or thiourea linker potentiated the anti-TB activity of 5-phenyl-3-isoxazolecarboxylic methyl esters indicating their role in enhanced permeability and execution of key interactions; (c) non-polar substituents on ureido/thioureido motif favoured anti-TB potency in contrast to detrimental effect shown by polar groups further strengthening the finding “enhanced lipophilicity associated increase in anti-TB potency”; (d) moreover, the observed diminished potency with hydrophobic bulky substituents (4u, 4v, 4x) supported that linear and compact pharmacophores were better suited for anti-TB potency; (e) in addition, isoxazole esters (4t as compared to 4td) were found to be essential for retention of anti-TB property; (f) reduction in potency with introduction of alkyl appendage to isoxazole esters (4ta as against 4t) suggested requirement of esters only for permeability, thus further strengthening the prodrug approach; (g) Diminished potency by the presence of adjacent substitution (4tc compared to 4t) to ureido/thioureido motif indicated the essential nature of later for target interaction.
All these observations were suggestive of prodrug attributes and pyrazinamide like action which is in agreement with previous studies [17].
Molecular docking studies
In order to further understand and correlate observed anti-TB potency, in silico evaluation was contemplated. MmpL3 was selected as the target protein considering the similarity in urea like core of target compounds to central structure of MmpL3 ligands indole-2-carboxamides [43, 48] and adamantyl urea derivatives [37]. To gain insight into the disparate activity, two compounds at opposite end of activity spectrum i.e., the most potent analogue 4t and poorly active compound 4v were subjected to molecular docking. Further, to explore and verify the prodrug/permeability theory, acid counterpart of optimum active compound i.e., 4te was also carried forward for molecular docking evaluation. Figure 5 illustrates predicted binding mode and detailed protein-inhibitor interactions of compound 4t, 4te, 4v and overlay of previously reported compound IV with 4te within active site of MmpL3.
Docking poses for compound 4t, pink stick (A); 4te, orange stick (B); 4v, green stick (C) and overlay of compound 4te and reference compound IV, slate blue stick (D) at the active site of protein MmpL3 (cyan cartoon; PDB code 6AJH). Hydrogen bonds shown as yellow dashes with distances in Å. Residue involved in H-bond and π-stalking interaction shown as teal and green cyan colour sticks, respectively
The 2D binding mode of aforementioned compounds along with cocrystal ligand are depicted in Fig. S1 of supplementary information. The molecular docking results along with major interactions for all these compounds with MmpL3 protein of Mycobacterium smegmatis are presented in Table S3 of Supplementary Information. From the molecular docking studies, it was observed that both 4t and 4te were well accommodated in active pocket of the protein, occupying similar pocket as that of co-crystal ligand AU-1235 and reference compound IV. However, compound 4t showed two hydrogen bond interactions with the active residues Asp-645 and Asp-256 in contrast to its carboxylic acid analogue 4te exhibiting identical key interaction as that of reference compound/co-crystal ligand with Asp-645 and Tyr-646 and hence showing far better binding affinity. The key interactions exhibited by 4te not only complimented to the rationale of design wherein, presumptions regarding urea/thiourea involving in key interactions got validated, but also further justified the prodrug hypothesis of the title compounds by which these elicited anti-TB activity as discussed above. Moreover, compound 4v also displayed similar interaction as that of cocrystal despite being poorly active against Mtb, further reaffirming the prodrug theory. i.e., irrespective of the interactions shown by isoxazole esters, the acid counterpart presumably elicits anti-TB activity. For compound 4te; the oxygen atom of urea moiety acts as hydrogen bond acceptor with residue Tyr-646 having distance 2.85 Å while both NH- groups acting as H-bond donor with Asp-645 having H-bond distance of 2.13 and 1.79 Å. Aryl fragment of ureido motif of 4te made one π-stalking interaction with Phe-649. Additionally, several hydrophobic interactions were observed for test compound and the active site residues, e.g., Ile-253, Tyr-257, Leu-259, Phe-260 (Fig. S1) that aided in stabilizing the binding of compound in the active site of MmpL3 which explained the requirement of hydrophobic/nonpolar groups to favour anti-TB potency.
Taken together, the molecular docking study corroborated the prodrug like attributes of the title compounds while speculating probable MmpL3 inhibition as one of the mechanisms of action. It has been debated that lipophilic non-aromatic moiety widely present in previously reported MmpL3 inhibitors assisted in enhanced penetration and increased affinity to target, leading to potent anti-TB activity. On the downside, these properties could also contribute to lower aqueous solubility and significant non-target binding and toxicity in plasma [49]. In this context, the prodrug approach, druglike properties and plausible MmpL3 inhibitory activity exhibited by the title compounds establishes these as promising anti-TB chemotypes.
In silico ADME/T studies
Drug-likeness, physiochemically key descriptors, and pharmacokinetically important properties of lead compounds 4t, 4tb and 5f were assessed by employing the QikProp programme of Schrödinger software. Table 3 depicts the significant computed ADME/T parameters and their recommended ranges. From the analysis of computed ADME/T parameters, it can be inferred that the lead compounds confirm to Lipinski's rule of five and has an acceptable range of physicochemical properties.
Conclusion
A library of urea-/thiourea-based 5-phenyl-3-isoxazolecarboxylic acid methyl ester derivatives have been conveniently synthesized and evaluated against Mtb H37Rv leading to a new potent and selective anti-TB chemotype exhibiting sub µg/mL activity. SAR findings demonstrated that mono- or disubstituted urea analogues having halogen or alkyl substitution were beneficial for potent activity whereas polar groups, bulky substituents were detrimental for potency. Reduction in potency with introduction of alkyl appendage to isoxazole esters suggested requirement of esters only for permeability. Urea moiety fared better in displaying anti-Mtb potency than its thiourea counterpart, indicating linear and compact pharmacophores were better suited for anti-TB potency. Isoxazole ester core is indispensable for activity considering obliteration in potency upon its alteration with triazole ester 4td or corresponding acid 4te. Lipophilicity was found firmly associated with anti-TB potency in a directly proportionate manner. All these observations were suggestive of prodrug attributes and pyrazinamide like action which is in agreement with previous literatures. The most active compounds of the series 4t and 5f exhibited MIC of 0.25 and 1 µg/mL respectively against susceptible Mtb H37Rv while halting the growth of DR strains with MIC of 0.5–4 µg/mL. Structural modification of 4t led to identification of compound 4tb displaying improved selectivity (CC50 > 40 µg/mL and SI > 80). To sum up, potentiation of anti-TB activity of isoxazole esters by introduction of urea/thiourea motif was successfully accomplished in this work. Overall, sub µg/mL potency against both susceptible and resistant Mtb strains besides being non-toxic establishes urea/thiourea variants of 5-phenyl-3-isoxazolecarboxylic acid methyl esters as a promising chemotype in pursuit of novel anti-TB agent. With apparent lack of isoxazole and urea-/thiourea-based pharmacophores as anti-infective drug candidates, the present outcome could certainly open up a roadmap for design and development of these scaffolds as prospective anti-TB agents.
Materials and methods
All the chemicals, reagents and starting materials were procured from commercial providers and were used as such. The monitoring of reactions was performed by TLC-MERCK pre-coated silica gel 60-F254 (0.5 mm) aluminium plates under UV light. 1H and 13C NMR spectra were obtained on Bruker Avance 500 MHz spectrometer using tetramethyl silane (TMS) as the internal standard and chemical shifts are reported in ppm. Chemical shifts are referenced to TMS (δ 0.00 for 1H NMR and 13C NMR) and corresponding solvents used for NMR recording CDCl3 (δ 7.26 for 1H NMR and 77.2 13C NMR) or DMSO/d6 (δ 2.50 for 1H NMR and 39.5 for 13C NMR). Spin multiplicities for 1H NMR are reported as s (singlet), brs (broad singlet), d (doublet), dd (double doublet), t (triplet) and m (multiplet). Unless and otherwise mentioned, values in 13C NMR are implicated as single Carbon while multiple Carbon is shown as ‘nC’ where n implies no. of Carbon. Coupling constant (J) values are reported in hertz (Hz). In 13C NMR of Fluorine containing compound, Carbon–Fluorine coupling is denoted by JCF. HRMS were determined with Agilent QTOF mass spectrometer 6540 series instrument and were performed in the ESI techniques at 70 eV. Column chromatography was performed using silica gel 60–120 or 100–200 mesh. Melting point was taken using Stuart® SMP30 apparatus.
Synthesis of intermediate anilines
General procedure for synthesis of isoxazole ester bearing aniline 3a–d
To an ice cold solution of appropriate diesters of oxalic acid (dimethyl oxalate or diethyl oxalate, 9.0 mmol, 1.5 equiv.) in dry THF was added sodium tertiary butoxide (21.2 mmol, 3.5 equiv.) under nitrogen atmosphere. After 10 min., a solution of substituted acetophenone 1a–c (6.0 mmol, 1 equiv.) in anhydrous THF was added dropwise to above suspension maintaining temp. below 0 °C. The resulting mixture was allowed to stir at room temperature for 1 h. After completion of the reaction (monitored by TLC), the reaction mixture was added to crushed ice and quenched with conc. HCl solution until acidic (pH 2–3); extracted with EtOAc and the combined organic layer was washed with brine, dried over Na2SO4, filtered, concentrated under reduced pressure to give 2,4-diketo ester intermediate 2a–d as light-yellow crystals; which were taken to the next step without further purification. The crude product obtained above was dissolved in appropriate solvent (MeOH or EtOH) and 1.2 equiv. of hydroxylamine hydrochloride was added. The solution was refluxed for 10–12 h. After attaining rt, excess solvent was minimized by vacuum evaporation. Upon cooling over ice, the precipitate formed was filtered, washed with cold MeOH/EtOH and oven dried to provide target nitro intermediates in good to excellent yield. A suspension of above nitro intermediate in 1:1 mixture of MeOH (or EtOH) and H2O was heated to 65 °C and sodium dithionite was gradually added until TLC shows complete consumption of starting material. The reaction mixture was extracted with EtOAc after removal of residual solvent. The combined organic layer was washed with brine, dried over Na2SO4, evaporated to yield corresponding amine intermediate 3a–d which were purified by recrystallization from chloroform and hexane. Please see Supporting information for characterization data of intermediate anilines 3a–d.
Procedure for synthesis of triazole ester containing aniline 7
3-Nitroaniline 6 (0.5 g, 3.62 mmol) was stirred in 6 N HCl (20 mL) and cooled to 0 °C in an ice-water bath followed by addition of 0.38 g NaNO2 (5.43 mmol, 1.5 equiv.) in 5 mL H2O dropwise and stirred for 30 min under cooling condition. A solution of 0.94 g NaN3 (21.30 mmol, 4 equiv.) in 7 mL H2O was added drop-wise and allowed to react for 1 h at rt. The resulting suspension was extracted with EtOAc and the organic layer was washed successively with saturated NaHCO3 and brine; dried over Na2SO4. The solvent was evaporated to give crude product 3-nitroazidobenzene (0.45 g) as white solid. The formed product was dissolved in DMF followed by the addition of ethyl acetoacetate (0.5 mL, 3.91 mmol, 1.2 equiv.) and Triethyl amine (0.9 mL, 6.52 mmol, 2 equiv.). The resulting reaction mixture was stirred at rt overnight. Upon completion, ice cold water was added and precipitate formed was filtered off and washed successively with cold water and then vacuum dried. The products were purified by recrystallization from EtOH to afford the desired compound as white solid. Obtained compound was dissolved in reaction grade ethyl acetate and purged with nitrogen thrice. 10 mol% Pd/C was added to the reaction mixture and was allowed to stir under H2 at rt. Upon completion (monitored by TLC Rf 0.2 in EtOAc:Hex 1:3), reaction mixture was celite filtered, filtrate washed with brine and evaporated to yield 7 as white powder in 78% (0.59 g, 2.14 mmol) yield. Please see Supporting information for characterization data of intermediate 7.
General procedure A for the synthesis of urea derivatives (4a–y)
Equimolar quantities (0.4 mmol) of substituted phenyl isocyanate or benzyl isocyanate and intermediate 3a (88 mg) was stirred in dry tetrahydrofuran for 16–18 h at room temperature under inert atmosphere. After completion of reaction (monitored by TLC), the solvent was removed in rota evaporator, then the residue was precipitated in acetonitrile and collected by filtration to give desired product 4a–y in 40–88% yield. All the newly synthesized compounds were characterized by 1H NMR, 13C NMR and HRMS (ESI). 1H NMR signals was also determined for some representative compounds (Supporting information). Please see Supporting Information for characterization data of compounds 4a–y.
General procedure B for the synthesis of thiourea derivatives (5a–o)
Equimolar quantities (0.4 mmol) of substituted phenyl isothiocyanate and intermediate 3a in dry acetonitrile was allowed to react for 16–18 h at room temp. under inert atmosphere. When 3a (88 mg) was treated with aliphatic isothiocyanates, additionally 2 equiv. of triethylamine was used as base under reflux for 18–20 h. Upon completion of reaction, as monitored by TLC, excess solvent was removed under vacuum and residue was purified by recrystallization form a mixture of dichloromethane and hexane or subjected to silica gel chromatography to furnish compounds 5a–o in 37–77% yield. All newly synthesized compounds were characterized by 1H NMR, 13C NMR and HRMS (ESI). 1H NMR signals was also elucidated for some representative compounds (Supporting information). Please see Supporting Information for characterization data of compounds 5a–o.
Synthesis of structurally modified analogues (4ta–te)
Condensation of 3,4-dichlorophenyl isocyanate (0.4 mmol) with aniline intermediates 3b–d or 7 (0.4 mmol) by employing general procedure A led to the formation of 4ta–td respectively in 74–85% yield.
Isoxazole carboxylic acid analogue 4te was obtained by basic hydrolysis of 4t. It was accomplished using 5 equiv. of LiOH monohydrate (51.6 mg, 1.23 mmol) in a mixture of 4t (100 mg, 0.25 mmol) in THF: H2O 9:1 at rt. After completion of reaction, volatile solvent was evaporated, residue diluted with cold water. pH of the solution formed was adjusted to 3–4 by dil. HCl. Resultant precipitate was vacuum filtered and oven dried to afford 4te as white solid, Yield 92% (89 mg, 0.23 mmol). Please see Supporting information for characterization data of compounds 4ta–te.
Antibiotic susceptibility testing against mycobacteria
Antimycobacterial susceptibility testing of test compounds was carried out using broth microdilution assay [50]. 10 mg/mL stock solutions of test and control compounds were prepared in DMSO and stored in − 20 °C. Mycobacterial cultures were inoculated in Middlebrook 7H9 enriched (Difco, Becton, NJ, USA) media supplemented with 10% ADC-Tween-80 (Bovine Serum Albumin, Dextrose, 0.2% glycerol and 0.05% Tween-80) and OD600 of cultures was measured, followed by dilution to achieve ~ 106 CFU/mL. The newly synthesized compounds were tested from 64 to 0.03 mg/mL in twofold serial diluted fashion with 2.5 mL of each concentration added per well of a 96-well round bottom microtiter plate. Later, 97.5 mL of bacterial suspension was added to each well containing the test compound along with appropriate controls. Presto blue (Thermo Fisher, USA) resazurin-based dye was used for the visualized identification of active compounds [51]. MIC of active compound was determined as lowest concentration of compound that inhibited visible growth after incubation period. The MIC results evaluated were obtained from one among the conc. of 0.03, 0.06, 0.12, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64 and > 64 mg/mL of test solution. MIC was detected on basis of visual colour change of culture medium from blue to pink. The lowest conc. that prevents this colour change was recorded as MIC, the determination of which was performed in triplicate and consistent MIC value obtained was reported as final analysed MIC. i.e., the MIC result reported is the one obtained twice or thrice out of three times experiment. Average of the thrice replicate was not taken as MIC, as it would represent unjustified concentrations. The MIC plates were incubated at 37 °C for 7 days for Mtb and 48 h for other mycobacterial pathogens.
Antibiotic susceptibility testing against bacterial pathogen panel
Antibiotic susceptibility testing was carried out on the newly synthesized compounds by determining the Minimum Inhibitory Concentration (MIC) with reference to the standard CLSI guidelines [52]. MIC is defined as the minimum concentration of compound at which visible bacterial growth is inhibited. Bacterial cultures were grown in Mueller–Hinton cation supplemented broth (CAMHB). Optical density (OD600) of the cultures was measured, followed by dilution for ~ 106 CFU/mL. This inoculum was added into a series of test wells in a microtiter plate that contained various concentrations of compound under test ranging from 64 to 0.03 µg/mL. Controls i.e., cells alone and media alone (without compound + cells) and levofloxacin used as a reference standard. Plates were incubated at 37 °C for 16–18 h followed by observations of MIC values by the absence or presence of visible growth. For each compound, MIC determinations were performed independently thrice using duplicate samples each time.
Cell cytotoxicity assay
The active newly synthesized compounds were screened for their cell toxicity against Vero cells using MTT assay [53]. Doxorubicin was taken as a positive control and experiment was performed in triplicate. ~ 103 cells/ well were seeded in 96 well plate and incubated at 37 °C with a 5% CO2 atmosphere. After 24 h, compound was added ranging from 100 to 5 mg/L and incubated for 72 h at 37 °C with 5% CO2 atmosphere. After the incubation was over, MTT was added at 5 mg/L in each well, incubated at 37 °C for further 4 h, residual medium was discarded, 0.1 mL of DMSO was added to solubilise the formazan crystals and OD was taken at 540 nm for the calculation of CC50. CC50 is defined as the lowest concentration of compound which leads to a 50% reduction in cell viability.
Molecular docking studies
To rationalize the experimental results obtained, molecular docking studies were undertaken on representative compounds 4t, 4te, 4v, reference compound IV and co-crystallized ligand compound III (AU-1235) on the active site of Mycobacterial membrane protein Large 3 (MmpL3) by employing GLIDE docking module of Schrodinger suite 2020–3 [54, 55]. The protein crystal structure of MmpL3 in complex with AU-1235 was retrieved from the RCSB Protein Data Bank (PDB code: 6AJH, resolution 2.82 Å). Following this, protein was prepared using Protein Preparation Wizard of Maestro. Missing amino acid residues and side chains were added using Prime module of Maestro. The bound co-crystallized ligand was used to define the active site. All ligand molecules were built and optimized using Maestro Molecule Builder and OPLS-3e force field in LigPrep modules of Schrodinger software. Further, the prepared ligands were docked at active site of MmpL3. Docking interactions were visualized by Pymol.
References
World Health Organization (2021). Global Tuberculosis Report. World Health Organization, Geneva. https://www.who.int/publications/i/item/9789240037021. Accessed 05 Jul 2022
Stephanie F, Saragih M, Tambunan USF (2021) Recent progress and challenges for drug-resistant tuberculosis treatment. Pharmaceutics 13(5):592. https://doi.org/10.3390/pharmaceutics13050592
Dheda K, Perumal T, Moultrie H, Perumal R, Esmail A, Scott AJ et al (2022) The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir Med 10(6):603–622. https://doi.org/10.1016/S2213-2600(22)00092-3
Bansal R, Sharma D, Singh R (2018) Tuberculosis and its treatment: an overview. Mini Rev Med Chem 18(1):58–71. https://doi.org/10.2174/1389557516666160823160010
Kumar A, Chettiar S, Parish T (2017) Current challenges in drug discovery for tuberculosis. Expert Opin Drug Discov 12(1):1–4. https://doi.org/10.1080/17460441.2017.1255604
Dartois VA, Rubin EJ (2022) Anti-tuberculosis treatment strategies and drug development: challenges and priorities. Nat Rev Microbiol. https://doi.org/10.1038/s41579-022-00731-y
Kumar V, Patel S, Jain R (2018) New structural classes of antituberculosis agents. Med Res Rev 38(2):684–740. https://doi.org/10.1002/med.21454
Global New TB Drug Pipeline (2022) Working group on new TB drugs. https://www.newtbdrugs.org/pipeline/clinical. Accessed 05 Jul 2022
Fernandes GFS, Thompson AM, Castagnolo D, Denny WA, Dos Santos JL (2022) Tuberculosis drug discovery: challenges and new horizons. J Med Chem 65(11):7489–7531. https://doi.org/10.1021/acs.jmedchem.2c00227
Oh S, Trifonov L, Yadav VD, Barry CE 3rd, Boshoff HI (2021) Tuberculosis drug discovery: a decade of hit assessment for defined targets. Front Cell Infect Microbiol 11:611304. https://doi.org/10.3389/fcimb.2021.611304
Dalberto PF, de Souza EV, Abbadi BL, Neves CE, Rambo RS, Ramos AS et al (2020) Handling the hurdles on the way to anti-tuberculosis drug development. Front Chem. https://doi.org/10.3389/fchem.2020.586294
Wellington S, Hung DT (2018) The expanding diversity of mycobacterium tuberculosis drug targets. ACS Infect Dis 4(5):696–714. https://doi.org/10.1021/acsinfecdis.7b00255
Fan L, Wu X, Jin C, Li F, Xiong S, Dong Y (2018) MptpB promotes mycobacteria survival by inhibiting the expression of inflammatory mediators and cell apoptosis in macrophages. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2018.00171
Beresford NJ, Mulhearn D, Szczepankiewicz B, Liu G, Johnson ME, Fordham-Skelton A et al (2009) Inhibition of MptpB phosphatase from Mycobacterium tuberculosis impairs mycobacterial survival in macrophages. J Antimicrob Chemother 63(5):928–936. https://doi.org/10.1093/jac/dkp031
Tan LP, Wu H, Yang P-Y, Kalesh KA, Zhang X, Hu M et al (2009) High-throughput discovery of Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB) inhibitors using click chemistry. Org Lett 11(22):5102–5105. https://doi.org/10.1021/ol9023419
Vickers CF, Silva APG, Chakraborty A, Fernandez P, Kurepina N, Saville C et al (2018) Structure-based design of MptpB inhibitors that reduce multidrug-resistant Mycobacterium tuberculosis survival and infection burden in vivo. J Med Chem 61(18):8337–8352. https://doi.org/10.1021/acs.jmedchem.8b00832
Lilienkampf A, Pieroni M, Franzblau SG, Bishai WR, Kozikowski AP (2012) Derivatives of 3-isoxazolecarboxylic acid esters: a potent and selective compound class against replicating and nonreplicating Mycobacterium tuberculosis. Curr Top Med Chem 12(7):729–734. https://doi.org/10.2174/156802612799984544
Nepali K, Lee H-Y, Liou J-P (2019) Nitro-group-containing drugs. J Med Chem 62(6):2851–2893. https://doi.org/10.1021/acs.jmedchem.8b00147
Sahoo SK, Rani B, Gaikwad NB, Ahmad MN, Kaul G, Shukla M et al (2021) Synthesis and structure–activity relationship of new chalcone linked 5-phenyl-3-isoxazolecarboxylic acid methyl esters potentially active against drug resistant Mycobacterium tuberculosis. Eur J Med Chem 222:113580. https://doi.org/10.1016/j.ejmech.2021.113580
Mao J, Yuan H, Wang Y, Wan B, Pak D, He R et al (2010) Synthesis and antituberculosis activity of novel mefloquine-isoxazole carboxylic esters as prodrugs. Bioorg Med Chem Lett 20(3):1263–1268. https://doi.org/10.1016/j.bmcl.2009.11.105
Mao J, Yuan H, Wang Y, Wan B, Pieroni M, Huang Q et al (2009) From serendipity to rational antituberculosis drug discovery of mefloquine-isoxazole carboxylic acid esters. J Med Chem 52(22):6966–6978. https://doi.org/10.1021/jm900340a
Lilienkampf A, Pieroni M, Wan B, Wang Y, Franzblau SG, Kozikowski AP (2010) Rational design of 5-phenyl-3-isoxazolecarboxylic acid ethyl esters as growth inhibitors of Mycobacterium tuberculosis a potent and selective series for further drug development. J Med Chem 53(2):678–688. https://doi.org/10.1021/jm901273n
Lilienkampf A, Mao J, Wan B, Wang Y, Franzblau SG, Kozikowski AP (2009) Structure–activity relationships for a series of quinoline-based compounds active against replicating and nonreplicating Mycobacterium tuberculosis. J Med Chem 52(7):2109–2118. https://doi.org/10.1021/jm900003c
Pieroni M, Lilienkampf A, Wan B, Wang Y, Franzblau SG, Kozikowski AP (2009) Synthesis, biological evaluation, and structure–activity relationships for 5-[(E)-2-arylethenyl]-3-isoxazolecarboxylic acid alkyl ester derivatives as valuable antitubercular chemotypes. J Med Chem 52(20):6287–6296. https://doi.org/10.1021/jm900513a
Kumar-Sahoo S, Naiyaz-Ahmad M, Kaul G, Nanduri S, Dasgupta A, Chopra S et al (2022) Exploration of isoxazole-carboxylic acid methyl ester based 2-substituted quinoline derivatives as promising antitubercular agents. Chem Biodivers. https://doi.org/10.1002/cbdv.202200324
Huang Q, Mao J, Wan B, Wang Y, Brun R, Franzblau SG et al (2009) Searching for new cures for tuberculosis: design, synthesis, and biological evaluation of 2-methylbenzothiazoles. J Med Chem 52(21):6757–6767. https://doi.org/10.1021/jm901112f
Changtam C, Hongmanee P, Suksamrarn A (2010) Isoxazole analogs of curcuminoids with highly potent multidrug-resistant antimycobacterial activity. Eur J Med Chem 45(10):4446–4457. https://doi.org/10.1016/j.ejmech.2010.07.003
Azzali E, Machado D, Kaushik A, Vacondio F, Flisi S, Cabassi CS et al (2017) Substituted N-phenyl-5-(2-(phenylamino)thiazol-4-yl)isoxazole-3-carboxamides are valuable antitubercular candidates that evade innate efflux machinery. J Med Chem 60(16):7108–7122. https://doi.org/10.1021/acs.jmedchem.7b00793
Ronchetti R, Moroni G, Carotti A, Gioiello A, Camaioni E (2021) Recent advances in urea- and thiourea-containing compounds: focus on innovative approaches in medicinal chemistry and organic synthesis. RSC Med Chem. https://doi.org/10.1039/D1MD00058F
Ghosh AK, Brindisi M (2020) Urea derivatives in modern drug discovery and medicinal chemistry. J Med Chem 63(6):2751–2788. https://doi.org/10.1021/acs.jmedchem.9b01541
Doğan ŞD, Gündüz MG, Doğan H, Krishna VS, Lherbet C, Sriram D (2020) Design and synthesis of thiourea-based derivatives as Mycobacterium tuberculosis growth and enoyl acyl carrier protein reductase (InhA) inhibitors. Eur J Med Chem 199:112402. https://doi.org/10.1016/j.ejmech.2020.112402
Konduri S, Pogaku V, Prashanth J, Siva Krishna V, Sriram D, Basavoju S et al (2021) Sacubitril-based urea and thiourea derivatives as novel inhibitors for anti-tubercular against dormant tuberculosis. ChemistrySelect 6(16):3869–3874. https://doi.org/10.1002/slct.202004724
Konduri S, Bhargavi D, Prashanth J, Krishna VS, Sriram D, Rao KP (2021) Design and synthesis of “chloropicolinate amides and urea derivatives” as novel inhibitors for Mycobacterium tuberculosis. ACS Omega 6(2):1657–1667. https://doi.org/10.1021/acsomega.0c05690
Malapati P, Krishna VS, Nallangi R, Srilakshmi RR, Sriram D (2018) Identification and development of benzoxazole derivatives as novel bacterial glutamate racemase inhibitors. Eur J Med Chem 145:23–34. https://doi.org/10.1016/j.ejmech.2017.12.088
Brunner K, Maric S, Reshma RS, Almqvist H, Seashore-Ludlow B, Gustavsson A-L et al (2016) Inhibitors of the cysteine synthase CysM with antibacterial potency against dormant Mycobacterium tuberculosis. J Med Chem 59(14):6848–6859. https://doi.org/10.1021/acs.jmedchem.6b00674
Zheng H, Williams JT, Aleiwi B, Ellsworth E, Abramovitch RB (2020) Inhibiting Mycobacterium tuberculosis DosRST signaling by targeting response regulator DNA binding and sensor kinase heme. ACS Chem Biol 15(1):52–62. https://doi.org/10.1021/acschembio.8b00849
Brown JR, North EJ, Hurdle JG, Morisseau C, Scarborough JS, Sun D et al (2011) The structure–activity relationship of urea derivatives as anti-tuberculosis agents. Bioorg Med Chem 19(18):5585–5595. https://doi.org/10.1016/j.bmc.2011.07.034
Grzegorzewicz AE, Pham H, Gundi VA, Scherman MS, North EJ, Hess T et al (2012) Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat Chem Biol 8(4):334–341. https://doi.org/10.1038/nchembio.794
Shen HC, Hammock BD (2012) Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J Med Chem 55(5):1789–1808. https://doi.org/10.1021/jm201468j
Scherman MS, North EJ, Jones V, Hess TN, Grzegorzewicz AE, Kasagami T et al (2012) Screening a library of 1600 adamantyl ureas for anti-Mycobacterium tuberculosis activity in vitro and for better physical chemical properties for bioavailability. Bioorg Med Chem 20(10):3255–3262. https://doi.org/10.1016/j.bmc.2012.03.058
North EJ, Scherman MS, Bruhn DF, Scarborough JS, Maddox MM, Jones V et al (2013) Design, synthesis and anti-tuberculosis activity of 1-adamantyl-3-heteroaryl ureas with improved in vitro pharmacokinetic properties. Bioorg Med Chem 21(9):2587–2599. https://doi.org/10.1016/j.bmc.2013.02.028
Motamen S, Quinn RJ (2020) Analysis of approaches to anti-tuberculosis compounds. ACS Omega 5(44):28529–28540. https://doi.org/10.1021/acsomega.0c03177
Stec J, Onajole OK, Lun S, Guo H, Merenbloom B, Vistoli G et al (2016) Indole-2-carboxamide-based mmpl3 inhibitors show exceptional antitubercular activity in an animal model of tuberculosis infection. J Med Chem 59(13):6232–6247. https://doi.org/10.1021/acs.jmedchem.6b00415
Kondreddi RR, Jiricek J, Rao SP, Lakshminarayana SB, Camacho LR, Rao R et al (2013) Design, synthesis, and biological evaluation of indole-2-carboxamides: a promising class of antituberculosis agents. J Med Chem 56(21):8849–8859. https://doi.org/10.1021/jm4012774
Palomino JC, Martin A (2014) Drug resistance mechanisms in Mycobacterium tuberculosis. Antibiotics (Basel) 3(3):317–340. https://doi.org/10.3390/antibiotics3030317
Nguyen L (2016) Antibiotic resistance mechanisms in M tuberculosis: an update. Arch Toxicol 90(7):1585–1604. https://doi.org/10.1007/s00204-016-1727-6
Gygli SM, Borrell S, Trauner A, Gagneux S (2017) Antimicrobial resistance in Mycobacterium tuberculosis: mechanistic and evolutionary perspectives. FEMS Microbiol Rev 41(3):354–373. https://doi.org/10.1093/femsre/fux011
Onajole OK, Pieroni M, Tipparaju SK, Lun S, Stec J, Chen G et al (2013) Preliminary structure–activity relationships and biological evaluation of novel antitubercular indolecarboxamide derivatives against drug-susceptible and drug-resistant Mycobacterium tuberculosis strains. J Med Chem 56(10):4093–4103. https://doi.org/10.1021/jm4003878
Shao M, McNeil M, Cook GM, Lu X (2020) MmpL3 inhibitors as antituberculosis drugs. Eur J Med Chem 200:112390. https://doi.org/10.1016/j.ejmech.2020.112390
Wiegand I, Hilpert K, Hancock RE (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3(2):163–175. https://doi.org/10.1038/nprot.2007.521
Taneja NK, Tyagi JS (2007) Resazurin reduction assays for screening of anti-tubercular compounds against dormant and actively growing Mycobacterium tuberculosis, Mycobacterium bovis BCG and Mycobacterium smegmatis. J Antimicrob Chemother 60(2):288–293. https://doi.org/10.1093/jac/dkm207
Jorgensen JH, Hindler JF, Reller LB, Weinstein MP (2007) New consensus guidelines from the clinical and laboratory standards institute for antimicrobial susceptibility testing of infrequently isolated or fastidious bacteria. Clin Infect Dis 44(2):280–286. https://doi.org/10.1086/510431
van Meerloo J, Kaspers GJ, Cloos J (2011) Cell sensitivity assays: the MTT assay. Methods Mol Biol 731:237–245. https://doi.org/10.1007/978-1-61779-080-5_20
Yang X, Hu T, Yang X, Xu W, Yang H, Guddat LW et al (2020) Structural basis for the inhibition of mycobacterial MmpL3 by NITD-349 and SPIRO. J Mol Biol 432(16):4426–4434. https://doi.org/10.1016/j.jmb.2020.05.019
Zhang B, Li J, Yang X, Wu L, Zhang J, Yang Y et al (2019) Crystal structures of membrane transporter MmpL3, an anti-TB drug target. Cell 176(3):636–48.e13. https://doi.org/10.1016/j.cell.2019.01.003
Acknowledgements
SKS, OO, SM, PS are thankful to Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Govt. of India, New Delhi for award of NIPER fellowship. GK thanks DST-INSPIRE and MNA is grateful to Council of Scientific and Industrial Research (CSIR), Govt. of India for award of SRF fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sahoo, S.K., Ommi, O., Maddipatla, S. et al. Isoxazole carboxylic acid methyl ester-based urea and thiourea derivatives as promising antitubercular agents. Mol Divers 27, 2037–2052 (2023). https://doi.org/10.1007/s11030-022-10543-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10543-0