Abstract
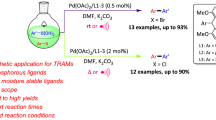
Petasis aryl and allyl borations were accomplished using substituted ninhydrins, boronic acids or 2-allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane and 1,2-aminophenols in Hexafluoroisopropanol (HFIP) without any catalysts to synthesize different aryl and allyl derivatives of ninhydrins. The nature of substitution in the boronic acids and 1,2-amino phenols was the key factor in determining the diastereo-regioselectivity and the type of product distributions. The products were isolated and characterized by HMBC, HSQC, 1H, 13C NMR experiments and X-ray single crystallographic analysis. A probable reaction pathway involves in situ formation of acyclic and cyclic ninhydrin-amino alcohol adducts, with the positioned hydroxyl group determining the stereo-regioselective outcome via tetracoordinated boron intermediates.
Graphical abstract
A metal free diastereo- and regioselective Petasis aryl and allyl boration of ninhydrins.










Similar content being viewed by others
References
Petasis NA, Akritopoulou I (1993) The boronic acid mannich reaction: a new method for the synthesis of geometrically pure allylamines. Tetrahedron Lett 34:583–586. https://doi.org/10.1016/S0040-4039(00)61625-8
Petasis NA, Zavialov I (1997) A new and practical synthesis of α-amino acids from alkenyl boronic acids. J Am Chem Soc 119:445–446. https://doi.org/10.1021/ja963178n
Petasis NA, Boral S (2001) One-step three-component reaction among organoboronic acids, amines and salicylaldehydes. Tetrahedron Lett 42:539–542. https://doi.org/10.1016/S0040-4039(00)02014-1
Muncipinto G, Moquist PN, Schreiber SL, Schaus SE (2011) Catalytic diastereoselective petasis reactions. Angew Chem Int Ed 50:8172–8175. https://doi.org/10.1002/anie.201103271
Li Y, Xu M-H (2012) Lewis acid promoted highly diastereoselective PetasisBorono-Mannich reaction: efficient synthesis of optically active β, γ-unsaturated α-amino acids. Org Lett 14:2062–2065. https://doi.org/10.1021/ol300581n
Chevis PJ, Wangngae S, Thaima T, Carroll AW, Willis AC, Pattarawarapan M, Pyne SG (2019) Highly diastereoselective synthesis of enantioenriched anti-α-allyl-β-fluoroamines. ChemComm 55:6050–6053. https://doi.org/10.1039/C9CC02765C
Jiang Y, Schaus SE (2017) Asymmetric Petasis Borono-Mannich allylation reactions catalyzed by chiral biphenols. Angew Chem Int Ed 56:1544–1548. https://doi.org/10.1002/anie.201611332
Kavouris J, Kavouris K, Wambua V M, Demerzhan R, Moquist P, Vetticatt M, Schaus S (2020) Chiral amino alcohols via catalytic enantioselective Petasis Borono-Mannich Reactions. Chem Catal. https://doi.org/10.26434/chemrxiv.12479654.v1
Jiang Y, Schaus SE, Thomson RJ (2017) Asymmetric traceless PetasisBorono-Mannich reactions of enals: reductive transposition of allylic diazenes. Angew Chem Int Ed 56:16631–16635. https://doi.org/10.1002/anie.201708784
Han W-Y, Zuo J, Zhang X-M, Yuan W-C (2013) Enantioselective Petasis reaction among salicylaldehydes, amines, and organoboronic acids catalyzed by BINOL. Tetrahedron 69:537–541. https://doi.org/10.1016/j.tet.2012.11.043
Han W-Y, Wu Z-J, Zhang X-M, Yuan W-C (2012) Enantioselective organocatalytic three-component petasis reaction among salicylaldehydes, amines, and organoboronic acids. Org Lett 14:976–979. https://doi.org/10.1021/ol203109a
Huo H-X, Duvall JR, Huanga M–Y, Hong R (2014) Catalytic asymmetric allylation of carbonyl compounds and imines with allylic boronates. Org Chem Front 1:303–320. https://doi.org/10.1039/C3QO00081H
Carrera DE (2017) The acid promoted Petasis reaction of organotrifluoroborates with imines and enamines. ChemComm 53:11185–11188. https://doi.org/10.1039/C7CC04397J
Yi J, Badir SO, Alam R, Molander GA (2019) Photoredox-catalyzed multicomponent petasis reaction with alkyltrifluoroborates. Org Lett 21:4853–4858. https://doi.org/10.1021/acs.orglett.9b01747
Petasis NA, Zavialov IA (1988) Highly stereocontrolled one-step synthesis of anti-β-amino alcohols from organoboronic acids, amines, and α-hydroxy aldehydes. J Am Chem Soc 120:11798–11799. https://doi.org/10.1021/ja981075u
Au CWG, Pyne SG (2006) Asymmetric synthesis of anti-1,2-amino alcohols via the Borono-Mannich reaction: a formal synthesis of (-)-swainsonine. J Org Chem 71:7097–7099. https://doi.org/10.1021/jo0610661
Thaima T, Pyne SG (2015) Regioselective and diastereoselective Borono-Mannich reactions with Pinacol allenylboronate. Org Lett 17:778–781. https://doi.org/10.1021/ol503424k
Yus M, González-Gómez JC, Foubelo F (2013) Diastereoselective allylation of carbonyl compounds and imines: application to the synthesis of natural products. Chem Rev 113:5595–5698. https://doi.org/10.1021/cr400008h
Carroll AW, Savaspun K, Willis AC, Hoshino M, Kato A, Pyne SG (2018) Total synthesis of natural hyacinthacine C5 and six related hyacinthacine C5 epimers. J Org Chem 83:5558–5576. https://doi.org/10.1021/acs.joc.8b00585
Bouillon ME, Pyne SG (2014) Diastereoselective concise syntheses of the polyhydroxylated alkaloids DMDP and DAB. Tetrahedron Lett 55:475–478. https://doi.org/10.1016/j.tetlet.2013.11.068
Ghosal P, Shaw AK (2012) A Chiron approach to aminocytitols by Petasis-Borono-Mannich reaction: formal synthesis of (+)-conduramine E and (−)-conduramine E. J Org Chem 77:7627–7632. https://doi.org/10.1021/jo300804d
Neto I, Andrade J, Fernandes AS, Reis CP, Salunke JK, Priimagi A, Candeias NR, Rijo P (2016) Multicomponent petasis-boronomannich preparation of alkylaminophenols and antimicrobial activity studies. Chem Med Chem 11:2015–2023. https://doi.org/10.1002/cmdc.201600244
Montalbano F, Cal PMSD, Carvalho MABR, Goncalves LM, Lucas SD, Guedes RC, Veiros LF, Moreira R, Gois PMP (2013) Discovery of new heterocycles with activity against human neutrophile elastase based on a boron promoted one-pot assembly reaction. Org Biomol Chem 11:4465–4472. https://doi.org/10.1039/C3OB40614H
Zhang X, Jiang W, Jacutin-Porte S, Glunz PW, Zou Y, Cheng X, Nirschi AH, Wurtz NR, Luettgen JM, Rendiana AR, Luo G, Harper TM, Wei A, Anumula R, CheneyD L, Knabb RM, Wong PC, WexlerR R, Priestley ES (2014) Design and synthesis of phenylpyrrolidine phenylglycinamides as highly potent and selective TF-FVIIa inhibitors. ACS Med Chem Lett 5:188–192. https://doi.org/10.1021/ml400453z
Rimpilainen T, Andrade J, Nunes A, Ntungwe E, Fernandes AS, Vale JR, Rodrigues J, Gomes JP, Rijo P, Candeias NR (2018) ACS Omega 3:16191–16202. https://doi.org/10.1021/acsomega.8b02381
Doan P, Karjalainen A, Chandraseelan JG, Sandberg O, Yli-Harja O, Rosholm T, Franzen R, Candeias NR, Kandhavelu M (2016) Synthesis and biological screening for cytotoxic activity of N-substituted indolines and morpholines. Eur J Med Chem 120:296–303. https://doi.org/10.1016/j.ejmech.2016.05.024
Natarajan K, Jesin CPI, Mercy AAH, Nandi GC (2021) A metal-free Petasis reaction towards the synthesis of N-(α-substituted)alkylsulfoximines/sulfonimidamides. Org Biomol Chem 19:7061–7065. https://doi.org/10.1039/D1OB01181B
Candeias NR, Montalbano F, Cal PMSD, Gois PMP (2010) Boronic acids and esters in the petasis-borono mannich multicomponent reaction. Chem Rev 110:6169–6093. https://doi.org/10.1021/cr100108k
Wu P, Nielsen TE (2018) Petasis three-component reactions for the synthesis of diverse heterocyclic scaffolds. Drug Discovery Today 29:27–33. https://doi.org/10.1016/j.ddtec.2018.06.010
Guerrera CA, Ryder TR (2016) The Petasis Borono-Mannich multicomponent reaction. ACS symposium series (boron reagents in synthesis) Chapter 9. 1236:275–311. https://doi.org/10.1021/bk-2016-1236.ch009
Ramadhar TR, Batey RA (2011) Recent advances in nucleophilic addition reactions of organoboronic acids and their derivatives to unsaturated C–N functionalities. Boronic acids: preparation and applications in organic synthesis Wiley-VCH, 2nd edn. 2:427–477. https://doi.org/10.1002/9783527639328.ch9
Carboni B, Berrée F (2013) Third component boronic acid (Petasis reaction), Müller T.J.; Multicomponent reactions. Sci. Synth. 1:219–259
Wu P, Givskov M, Nielsen T (2019) Reactivity and synthetic applications of multicomponent Petasis reactions. Chem Rev 119:11245–11290. https://doi.org/10.1021/acs.chemrev.9b00214
Hwang J, Borgelt L, Wu P (2020) Multicomponent Petasis reaction for the synthesis of functionalized 2-aminothiophenes and thienodiazepines. ACS Comb Sci 22:495–499. https://doi.org/10.1021/acscombsci.0c00173
Mandai H, Murota K, Suga S (2012) Studies on the Petasis reaction of 2-pyridinecarbaldehyde derivatives and its products. Heterocycles 85:1655–1669. https://doi.org/10.3987/COM-12-12500
Lenci E, Puglielli RB, Bucaletti E, Innocenti R, Trabocchi A (2020) A glucose-derived α-hydroxy aldehyde for the petasis reaction: facile access to polyfunctional δ-amino acids. Eur J Org Chem 2020:4227–4234. https://doi.org/10.1002/ejoc.202000600
Mandai H, Murota K, Sakai T (2010) An improved protocol for Petasis reaction of 2-pyridinecarbaldehydes. Tetrahedron Lett 51:4779–4782. https://doi.org/10.1016/j.tetlet.2010.07.039
Schlienger N, Bryce MR, Hansen TK (2000) Heterocylic aldehydes as novel components in the boronic Mannich reaction. Tetrahedron Lett 41:1303–1305. https://doi.org/10.1016/S0040-4039(99)02273-X
Chouguiat L, Boulcina R, Carboni B, Demonceau A, Debache A (2014) A new and efficient one-pot synthesis of 2-hydroxy-1,4-dihydroxybenzoxazines via a three-component Petasis reaction. Tetrahedron Lett 55:5124–5128. https://doi.org/10.1016/j.tetlet.2014.07.093
Wang Q, Finn MG (2000) 2H-chromenes from salicylaldehydes by a catalytic petasis reaction. Org Lett 2:4063–4065. https://doi.org/10.1021/ol006710r
Puentes CO, Kouznetsov V (2002) J Het Chem 39:595–614. https://doi.org/10.1002/jhet.5570390401
Yus M, González-Gómez JC, Foubelo F (2013) Diastereoselective allylation of carbonyl compounds and imines: application to the synthesis of natural products. Chem Rev 13:5595–5698. https://doi.org/10.1021/cr400008h
Ramadhar R, Batey RA (2011) Allylation of imines and their derivatives with organoboron reagents: stereocontrolled synthesis of homoallylic amines. Synthesis 9:1321–1346. https://doi.org/10.1055/S-0030-1258434
Kuramoto M, Tong C, Yamada K, Chiba T, Hayashi Y, Uemura D (1996) Halichlorine, an inhibitor of VCAM-1 induction from the marine sponge Halichondriaokadai Kadata. Tetrahedron Lett 37:3867–3870. https://doi.org/10.1016/0040-4039(96)00703-4
Trimurtulu G, Ohtani I, Patterson GML, Moore RE, Corbett TH, Valeriote FA, Demchiko L (1994) Total structures of cryptophycins, potent antitumor depsipeptides from the blue-green Alga Nostoc sp strain GSV 224. J Am Chem Soc 116:4729–4737. https://doi.org/10.1021/ja00090a020
Schmidt U, Schmidt J (1994) Synthesis the total synthesis of eponemycin. Synthesis 1994:300–304. https://doi.org/10.1055/s-1994-25464
Lin Z-W, Zhou Y, Zhao Z-N, Zhao Y, Liu J, Huang Y-Y (2019) 1,2-Amino alcohol-dependent Petasisallylboration for racemicand chiral homoallylamines. Org Chem Front 6:751–755. https://doi.org/10.1039/C8QO01428K
Yang Y, Cao Z-H, Zhou Y, Cheng F, Lin Z–W, Ou, Z, Yuan Y, Huang Y–Y (2018) Petasis-type gem-difluoroallylation reactions assisted by the neighboring hydroxyl group in amines. Org Lett 20:2585–2589. https://doi.org/10.1021/acs.orglett.8b00721
Tan Q, Wang X, Xiong Y, Zhao Z, Li L, Tang P, Zhang M (2017) Chiral amino alcohol accelerated and stereocontrolled allylboration of iminoisatins: highly efficient construction of adjacent quaternary stereogenic centers. Angew Chem Int Ed 56:4829–4833. https://doi.org/10.1002/anie.201700581
Sengupta A, Maity S, Mondal A, Ghosh P, Rudra S, Mukhopadhyay C (2019) Pseudo five component reaction towards densely functionalized spiro[indole-3,2′-pyrrole] by picric acid, an efficient syn-diastereoselective catalyst: insight into the diastereoselection on C(sp3)–C(sp3) axial conformation. Org Biomol Chem 17:1254–1265. https://doi.org/10.1039/C8OB02849D
Ziarani GM, Lashgari N, Azimian F, Kruger HG, Gholamzadeh P (2015) Ninhydrin in synthesis of heterocyclic compounds. ARKIVOC 4:1–139. https://doi.org/10.3998/ark.5550190.p008.905
Das S (2020) Recent applications of ninhydrin in multicomponent reactions. RSC Adv 10:18875–18906. https://doi.org/10.1039/D0RA02930K
Mousavi SH, Mohammadizadeh MR, Roshan Z, Jamaleddini A, Arimitsu S (2020) One-pot synthesis of spiro-isobenzofuran compounds via the sequential condensation/oxidation reaction of ninhydrin with 4-amino-1,2-naphthoquinones/2-amino-1,4-naphthoquinones under mild conditions. ACS Omega 5:18273–18288. https://doi.org/10.1021/acsomega.0c01934
Das S, Dutta A (2020) Ninhydrin adducts as valid synthon in organic synthesis: a review. Chem Select 5:11361–11377. https://doi.org/10.1002/slct.202003245
Okpekon T, Millot M, Champy P, Gleye C, Yolou S, Bories C, Loiseau P, Laurens LA, Hocquemiller R (2009) A novel 1-indanone isolated from Uvaria afzelii roots. Nat Prod Res 23:909–915. https://doi.org/10.1080/14786410802497240
Yang Y, Philips D, Pan S (2011) A concise synthesis of paucifloral f and related indanone analogues via palladium-catalyzed α-arylation. J Org Chem 76:1902–19065. https://doi.org/10.1021/jo102298p
Azuma T, Tanaka Y, Kikuzaki H (2008) Phenolic glycosides from Kaempferia parviflora. Phytochemistry 69:2743–2748. https://doi.org/10.1016/j.phytochem.2008.09.001
Ruchirawat S, Thasana N (2001) The first synthesis of Wrightiadione. Synth Commun 31:1765–1769. https://doi.org/10.1081/SCC-100104406
Zhang J, El-Shabrawy A-RO, El-Shanawany MA, Schiff PL Jr, Slatkin DJ (1987) New azafluorene alkaloids from oxandraxylopioides. J Nat Prod 50:800–806. https://doi.org/10.1021/np50053a005
Wang S, Kraus GA (2019) Annulation of 5-phenylthiobutenolides and first synthesis of (±)-Indanostatin. Synlett 30:353–355. https://doi.org/10.1055/s-0037-1611462
Sahu KB, Banerjee M, Ghosh S, Maity A, MondalS PR, Hazra A, Karmakar S, Samanta A, Mondal NB (2013) I2 catalyzed Friedel-Crafts alkylation reaction of substituted anilines with ninhydrin: formation of novel products and their antimicrobial evaluation. Med Chem Res 22:2023–2037. https://doi.org/10.1007/s00044-012-0202-z
Garrido F, Ibanez J, Gonalons E, Giraldez A (1975) Synthesis and laxative properties of some derivative esters of 3,3-bis-(4-hydroxyphenyl)-2-indolinone. Eur J Med Chem 10:143–146
Poupelin JP, Saint-Ruf G, Perche JC, Roussey JC, Laude B, Narcisse G, Bakri-Logeais F, Hubert F (1980) 2-Hydroxy-1,3-indandione derivatives. II. Condensation products of ninhydrin with polyphenols and their O-methylated derivatives. Eur J Med Chem 15:253–262
Prabhakar KR, Veerapur VP, Bansal P, Vipan KP, Reddy KM, Barik A, Reddy BKD, Reddanna P, Priyadarsini KI, Unnikrishnan MK (2006) Identification and evaluation of antioxidant, analgesic/anti-inflammatory activity of the most active ninhydrin–phenol adducts synthesized. Bioorg Med Chem 14:7113–7120. https://doi.org/10.1016/j.bmc.2006.06.068
Kashyap M, Das D, Preet R, Mohapatra P, Satapathy SR, Siddharth S, Kundu CN, Guchhait SK (2012) Scaffold hybridization in generation of indenoindolones as anticancer agents that induce apoptosis with cell cycle arrest at G2/M phase. Bioorg Med Chem Lett 22:2474–2479. https://doi.org/10.1016/j.bmcl.2012.02.007
Gogoi A, Das G (2014) NIR sensing of Zn(II) and subsequent dihydrogen phosphate detection by a benzothiazole functionalized ninhdrin based receptor. RSC Adv 4:55689–55695. https://doi.org/10.1039/C4RA10556G
Das S, Das P, Maity S, Ghosh P, Paul BK, Dutta A (2018) Benzimidazole-based polyheterocycles from ninhydrin: synthesis, X-ray crystal structure and photophysical property. J Mol Struct 1168:234–241. https://doi.org/10.1016/j.molstruc.2018.05.033
Das S, Fröhlich R, Pramanik A (2006) Synthesis and fluorescent properties of a new class of heterocycles of isoindole fused imidazoles with phenolic subunits. Org Lett 8:4263–4266. https://doi.org/10.1021/ol061520n
Song HS, Lee HJ, Kim HR, Ryu EK, Kim JN (1999) Friedel-Crafts type reactions of some activated cyclic ketones with phenolderivatives. Synth Commun 29:3303–3311. https://doi.org/10.1080/00397919908085958
Kundu S, Patra A, Pramanik A, (2004) Facile acid-catalyzed condensation of ninhydrin with enols and aromatic compounds and microwave enhanced condensation of ninhydrin with hydroxyl aromatic systems in solid state. Indian J Chem 43B:604–611
Kundu S, Das S, Pramanik A (2004) Theoretical studies of the acid-catalyzed condensation with aromatic compounds. Indian J Chem 43B:2212–2216
Na JE, Lee SS, Kim JN (2004) Design and synthesis of ninhydrin-based cyclophanes as potential neutral receptors for quaternary ammonium cations. Tetrahedron Lett 45:7435–7440. https://doi.org/10.1016/j.tetlet.2004.08.078
Das P, Maity S, Ghosh P, Dutta A, Das S (2020) Condensation of ninhydrin with phenols: regioselective formation of diverse organic scaffolds and crystal structure studies. J Mol Struct 1202:127260. https://doi.org/10.1016/j.molstruc.2019.127260
Taylor BM, Joullié MM (1998) Reaction of 1,2-indanedione with 3,5-dimethoxyaniline. Tetrahedron 54:5121–15126. https://doi.org/10.1016/S0040-4020(98)00911-9
Black D, Bowyer MC, Condie GC, Craig DC, Kumar N (1994) Reactions of ninhydrin with activated anilines: formation of indole derivatives. Tetrahedron 50:10983–10994. https://doi.org/10.1016/S0040-4020(01)85709-4
Friedman M (1967) Mechanism of the ninhydrin reaction II. Preparation and spectral properties of reaction products from primary aromatic amines and ninhydrin hydrate. Can J Chem 45:2271–2275. https://doi.org/10.1139/v67-369
Schönberg A, Singer E (1977) Konstitutionsermittlung der Reaktionsprodukte von Ninhydrinmit 2-aminophenol bzw 2-Aminothiophenol. ChemBer 110:3954–3958. https://doi.org/10.1002/cber.19771101229
Simakov VI, Kurbatov SV, Borbulevych OY, Antipin MY, OlekhnovichL P (2001) Structures of condensation products of ortho-aminophenols with ninhydrin. Russian Chem Bull Int Ed 50:1064–1067. https://doi.org/10.1023/A:1011333722238
Roth HJ, Kok W (1976) ZurKenntnis der Ninhydrin-Reaktion, 4 Mitt. Reaktionenmit 2-und 4-Dimethylaminoanilin. Arch Pharm Pharm Med Chem 309:92–98. https://doi.org/10.1002/ardp.19763090203
Sakuma H, Natsumi S, Hidetsugu W, Hiroshi M, Keiji K (2008) Dynamic behavior of cyclic hemiac et al sof 2-hydroxy-2-(2-hydroxyphenyl)-1,3-indandione derivatives. Chem Lett 37:696–697. https://doi.org/10.1246/cl.2008.696
Nanda KK, Trotter BW (2005) Diastereoselective Petasis Mannich reactions accelerated by hexfluoroisopropanol: a pyrrolidone-derived arylglycine synthesis. Tetrahedron Lett 46:2025–2028. https://doi.org/10.1016/j.tetlet.2005.01.151
Marminon C, Nacereddine A, Bouaziz Z, Nebois P, Jose J, Borgne ML (2015) Microwave-assisted oxidation of indan-1-ones into ninhydrins. Tetrahedron Lett 56:1840–1842. https://doi.org/10.1016/j.tetlet.2015.02.086
Jong JAW, Moret M-E, Verhaar MC, Hennink WE, Gerritsen KGF, van Nostrum CF (2018) Effect of substituents on the reactivity of ninhydrin with urea. Chem Select 3:1224–1229. https://doi.org/10.1002/slct.201800040
Acknowledgements
We gratefully acknowledge The University of Calcutta and TCG Lifesciences Pvt. Ltd., India for this collaborative research. We also like to thank CAS-V (UGC), DST-FIST, and DST-PURSE, Department of Chemistry, University of Calcutta for funding as departmental projects.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sengupta, A., Maity, S., Saha, P. et al. Diastereo- and regioselective petasis aryl and allyl boration of ninhydrins towards synthesis of functionalized indene-diones and dihydrobenzoindeno-oxazin-ones. Mol Divers 27, 1385–1400 (2023). https://doi.org/10.1007/s11030-022-10496-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10496-4