Abstract
A detailed computational analysis of acridine derivatives viz. acridone, 9-amino acridine hydrochloride hydrate, proflavin, acridine orange and acridine yellow is done in terms of conceptual density functional theory (CDFT). CDFT-based global descriptors—ionization potential, electron affinity, HOMO–LUMO gap, hardness, softness, electronegativity and electrophilicity index of acridine derivatives for ground state as well as excited state are estimated with the help of different hybrid functionals B3LYP/6-31G (d, p), B3LYP/6-311G (d, p), B3LYP/DGDZVP and B3LYP/LANL2DZ. Acridine derivatives show higher values of ionization potential and electron affinity in excited state as compared to ground state, indicating that these compounds are willing to accept electrons in excited state rather than donating electron. Acridone shows the maximum HOMO–LUMO energy gap in ground and excited state which implies that one-way electron transfer is most feasible with this compound. Our computed results emphasize the pronounced electron acceptor behaviour of the acridine derivatives in the excited state which has already been experimentally verified. It is observed that the trend in the computed values of the descriptors is not much improved on refinement of the basis set.
Graphical abstract








Similar content being viewed by others
References
Lerman LS (1961) Structural considerations in the interaction of DNA and acridines. J Mol Biol 3(1):18–30. https://doi.org/10.1016/S0022-2836(61)80004-1
Lerman LS (1963) The structure of the DNA-acridine complex. Proc Natl Acad Sci USA 49(1):94–102. https://doi.org/10.1073/pnas.49.1.94
Nakatani K, Shirai J, Sando S, Saito I (1997) Dibenzoyldiazomethane-acridine conjugate: a novel DNA photofootprinting agent. Tetrahedron Lett 38(34):6047–6050. https://doi.org/10.1016/S0040-4039(97)01357-9
Baruah H, Day CS, Wright MW, Bierbach U (2004) Metal-intercalator-mediated self-association and one-dimensional aggregation in the structure of the excised major DNA adduct of a platinum-acridine agent. J Am Chem Soc 126(14):4492–4493. https://doi.org/10.1021/ja038592j
Miles S, Callow P, Tiexeira S, Gan Y, Denny W, Cardin C, Forsyth T (2006) Structural studies on acridine derivatives binding to telomeric DNA. Phys B 385:845–847. https://doi.org/10.1016/j.physb.2006.05.122
Martins C, Gunaratnam M, Stuart J, Makwana V, Greciano O, Reszeka AP, Kelland LR, Neidle S (2007) Structure-based design of benzylamino-acridine compounds as G-quadruplex DNA telomere targeting agents. Bioorg Med Chem Lett 17(8):2293–2298. https://doi.org/10.1016/j.bmcl.2007.01.056
Campbell NH, Parkinson GN, Reszka AP, Neidle S (2008) Structure basis of DNA quadruplex recognition by an acridine drug. J Am Chem Soc 130(21):6722–6724. https://doi.org/10.1021/ja8016973
Sparapani S, Haider SM, Doria F, Gunaratnam M, Neidle S (2010) Rational design of acridine-based ligands with selectivity for human telomeric quadruplexes. J Am Chem Soc 132(35):12263–12272. https://doi.org/10.1021/ja1003944
Fernandez MJ, Wilson B, Palacios M, Rodrigo MM, Gant KB, Lorente A (2007) Copper-activated DNA photocleavage by a pyridine-linked bis-acridine intercalator. Bioconjugate Chem 18(1):121–129. https://doi.org/10.1021/bc0601828
Joseph J, Eldho NV, Ramaiah D (2003) Control of electron-transfer and DNA binding properties by the tolyl spacer group in viologen linked acridines. J Phys Chem B 107(18):4444–4450. https://doi.org/10.1021/jp027248q
Kuzuya A, Komiyama M (2000) Non-covalent ternary systems (DNA-acridine hybrid/DNA/lanthanide(III)) for efficient and site-selective RNA scission. Chem Commun. https://doi.org/10.1039/B006772P
Kuzuya A, Mizoguchi R, Morisawa F, Machida K, Komiyama M (2002) Metal ion-induced site-selective RNA hydrolysis by use of acridine bearing oligonucleotide as cofactor. J Am Chem Soc 124(24):6887–6894. https://doi.org/10.1021/ja025653p
Kuzuya A, Machida K, Mizoguchi R, Komiyama M (2002) Conjugation of various acridines to DNA for site-selective RNA scission by lanthanide ion. Bioconjugate Chem 13(2):365–369. https://doi.org/10.2021/bc015573v
Kuzuya A, Machida K, Komiyama M (2002) A highly acidic acridine for efficient site-selective activation of RNA leading to an eminent ribozyme mimic. Tetrahedron Lett 43(46):8249–8252. https://doi.org/10.1016/S0040-4039(02)02017-8
Shi Y, Kuzuya A, Machida K, Komiyama M (2004) Crucial role of linker portion in acridine-bearing oligonucleotides for highly efficient site-selective RNA scission. Tetrahedron Lett 45(19):3703–3706. https://doi.org/10.1016/j.tetlet.2004.03.102
Tung CH, Wei Z, Leibowitz MJ, Stein S (1992) Design of peptide-acridine mimics of ribonuclease activity. Proc Natl Acad Sci USA 89(15):7114–7118. https://doi.org/10.1073/pnas.89.15.7114
Wallace RA, Kurtz AN, Niemann C (1963) Interaction of aromatic compounds with α-chymotrypsin. Biochemistry 2(4):824–836. https://doi.org/10.1021/bi00904a035
Bernhard SA, Lee BF (1964) Abstracts sixth international congress of biochemistry. IUB 32 p. 297 IV-9 New York, NY
Weiner H, Koshland DE Jr (1965) Comparative binding properties of native and anhydro chymotrypsin determined by a competitive dialysis method. J Biol Chem 240(6):PC2764–PC2766. https://doi.org/10.1016/S0021-9258(18)97395-3
Glazer AN (1965) Spectral studies of the interaction of alpha-chymotrypsin and trypsin with proflavine. Proc Natl Acad Sci USA 54(1):171–176. https://doi.org/10.1073/pnas.54.1.171
Bernhard SA, Lee BF, Tashzian ZH (1966) On the interaction of the active site of α-chymotrypsin with chromophores: proflavin binding and enzyme conformation during catalysis. J Mol Biol 18(3):405–420. https://doi.org/10.1016/S0022-2836(66)80033-5
Hannun YA, Bell RM (1988) Aminoacridines, potent inhibitors of protein kinase C. J Biol Chem 263(11):5124–5131. https://doi.org/10.1016/S0021-9258(18)60688-X
Fritsch C, Goerz G, Ruzicka T (1998) Photodynamic therapy in dermatology. Arch Dermatol 134(2):207–214. https://doi.org/10.1001/archderm.134.2.207
Varnell ED, Kaufmen HE (1973) Photodynamic inactivation with proflavine: quantitative comparison with iodo-deoxyuridine. Infect Immun 7(4):518–519. https://doi.org/10.1128/iai.7.4.518-519.1973
Demeunynck M, Charmantray F, Martelli A (2001) Interest of acridine derivatives in the anticancer chemotherapy. Curr Pharm Des 7(17):1703–1724. https://doi.org/10.2174/1381612013397131
Denny WA (2002) Acridine derivatives as chemotherapeutic agents. Curr Med Chem 9(18):1655–1665. https://doi.org/10.2174/0929867023369277
Zhang B, Li X, Li B, Gao C, Jiang Y (2014) Acridine and its derivatives: a patent review (2009–2013). Expert Opin Ther Patents 24(6):647–664. https://doi.org/10.1517/13543776.2014.902052
Steiner UE, Ulrich T (1989) Magnetic field effects in chemical kinetics and related phenomena. Chem Rev 89(1):51–147. https://doi.org/10.1021/cr00091a003
Bhattacharyya K, Chowdhury M (1993) Environmental and magnetic field effects on exciplex and twisted charge transfer emission. Chem Rev 93(1):507–535. https://doi.org/10.1021/cr0017a022
Chakraborty B, Basu S (2009) Study of interaction of proflavin with trimethylamine in homogeneous and micellar media: photoinduced electron transfer probed by magnetic field effect. Chem Phys Lett 477(4–6):382–387. https://doi.org/10.1016/j.cplett.2009.07.018
Chakraborty B, Basu S (2010) Interaction of proflavin with aromatic amines in homogeneous and micellar media: photoinduced electron transfer probed by magnetic field effect. Chem Phys Lett 487(1–3):51–57. https://doi.org/10.1016/j.cplett.2010.01.013
Chakraborty B, Roy AS, Dasgupta S, Basu S (2010) Magnetic field effect corroborated with docking study to explore photoinduced electron transfer in drug-protein interaction. J Phys Chem A 114(51):13313–13325. https://doi.org/10.1021/jp109604a
Chakraborty B, Basu S (2010) Magnetic field effect on electron transfer reactions of acridine yellow with amines of varied structures in homogeneous medium. Chem Phys Lett 493(1–3):76–82. https://doi.org/10.1016/j.cplett.2010.05.016
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, Oxford. https://doi.org/10.1093/oso/9780195092769.001.0001
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105(26):7512–7516. https://doi.org/10.1021/ja00364a005
Parr RG, Szentpaly LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121(9):1922–1924. https://doi.org/10.1021/ja983494x
Chattaraj PK, Sarkar U, Roy DR (2007) Electronic structure principles and aromaticity. J Chem Educ 84(2):354. https://doi.org/10.1021/ed084p354
Ranjan P, Kumar P, Chakraborty T, Sharma M, Sharma S (2020) A study of structure and electronic properties of chalcopyrites semiconductor invoking density functional theory. Mater Chem Phys 241:122346. https://doi.org/10.1016/j.matchemphys.2019.122346
Ranjan P, Chakraborty T (2020) A comparative study of structure, stabilities and electronic properties of neutral and cationic [AuSin]λ and [Sin+1] λ (λ = 0, +1; n=1-12) nanoalloy clusters. Mat Today Commun 22:100832. https://doi.org/10.1016/j.mtcomm.2019.100832
Ranjan P, Chakraborty T (2019) Density functional approach: to study copper sulphide nanoalloy clusters. Acta Chim Slov 66(1):173–181. https://doi.org/10.17344/acsi.2018.4762
Ranjan P, Chakraborty T (2020) Structure and electronic properties of AunPt (n=1-8) nanoalloy clusters: the density functional theory study. J Nanopart Res 22:35. https://doi.org/10.1007/s11051-019-4745-5
Ranjan P, Chakraborty T (2020) Structure and optical properties of (CuAg)n (n=1-6) nanoalloy clusters within density functional theory framework. J Nanopart Res 22:280. https://doi.org/10.1007/s11051-020-05016-0
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, revision C.01. Gaussian, Inc., Wallingford
Fu A, Du D, Zhou Z (2003) Density functional theory study of vibrational spectra of acridine and phenazine. Spectrochim Acta Part A 59(2):245–253. https://doi.org/10.1016/S1386-1425(02)00169-5
Karmakar A, Banerjee S, Singh B, Mandal NC (2019) Study of hydrogen bonding interaction of acridine orange with different acceptor molecules by spectroscopic, theoretical and antimicrobial studies. J Mol Struct 1177:418–429. https://doi.org/10.1016/j.molstruc.2018.09.074
Zadykowicz B, Storoniak P (2017) Lattice energetics and thermochemistry of acridine derivatives and substituted acridinium trifluoromethanesulphonates. J Therm Anal Calorim 129:1613–1624. https://doi.org/10.1007/s10973-017-6306-4
Pereira E, Quental LD, Palma E, Oliveira MC, Mendes F, Raposinho P, Correia I, Lavrado J, Maria SD, Belchior A, Vaz P, Santos I, Paulo A (2017) Evaluation of acridine orange erivatives as DNA-targeted radiopharmaceuticals for auger therapy: influence of the radionuclide and distance to DNA. Sci Rep 7:42544. https://doi.org/10.1038/srep42544
Thorat KG, Tayade RP, Sekar N (2016) Acridine-1, 8 diones—a new class of thermally stable NLOpores: photophysical, (hyper)polarizability and TD-DFT studies. Opt Mater 62:306–319. https://doi.org/10.1016/j.optmat.2016.10.020
Wang X, Yue Y, Zhang Y, Wang Z, Liu J, Tang Q (2019) Probing the interaction of pepsin with imidacloprid via DFT calculation, spectroscopic approaches and molecular docking. J Mol Struct 1197:210–216. https://doi.org/10.1016/j.molstruc.2019.07.061
Vaddamanu M, Sathyanarayana A, Masaya Y, Sugiyama S, Kazuhisa O, Velappan K, Nandeshwar M, Hisano K, Tsutsumi O, Prabusankar G (2021) Acridine N-heterocyclic carbine gold(I) compounds: tuning from yellow to blue luminescence. Chem Asian J 16(5):521–529. https://doi.org/10.1002/asia.202001380
Chakraborty B, Basu S (2012) Magnetic field effect on photoinduced electron transfer associated with hydrogen bond formation in homogeneous medium. Appl Mag Res 42:5–15. https://doi.org/10.1007/s00723-011-0254-0
Chakraborty B, Mitra P, Basu S (2015) Spectroscopic exploration of drug–protein interaction: a study highlighting the dependence of the magnetic field effect on inter-radical separation distance formed during photoinduced electron transfer. RSC Adv 5:81533–81545. https://doi.org/10.1039/C5RA13575C
Chakraborty B, Sengupta C, Pal U, Basu S (2017) Acridone in a biological nanocavity: detailed spectroscopic and docking analyses of probing both the tryptophan residues of bovine serum albumin. New J Chem 41:12520–12534. https://doi.org/10.1039/C7N02454A
Mitra P, Chakraborty B, Basu S (2014) A spectroscopic investigation of the photophysical behaviour of 9-aminoacridine hydrochloride hydrate in presence of organic amines in homogeneous and heterogeneous media. J Luminesc 149:221–230. https://doi.org/10.1016/j.jlumin.2014.01.034
Mitra P, Chakraborty B, Basu S (2014) Exploring photoinduced electron transfer and excited-state proton transfer reactions involving 9-aminoacridine hydrochloride hydrate and methyl viologen using laser flash photolysis. Chem Phys Lett 6610–611(2014):108–114. https://doi.org/10.1016/j.cplett.2014.07.004
Parr RG, Chattaraj PK (1991) Principle of maximum hardness. J Am Chem Soc 113(5):1854–1855. https://doi.org/10.1021/ja00005a072
Chattaraj PK, Lee H, Parr RG (1991) HSAB principle. J Am Chem Soc 113(5):1855–1856. https://doi.org/10.1021/ja00005a073
Bose A, Dey D, Basu S (2007) Structure-dependent switchover of reaction metods: a laser flash photolysis and magnetic field effect study. J Photochem Photobiol A 186(2–3):130–134. https://doi.org/10.1016/j.photochem.2006
Bose A, Sarkar AK, Basu S (2009) Role of sugar in controlling reaction pathways: a study with thymine and thymidine. J Lumin 129(10):1186–1191. https://doi.org/10.1016/j.jlumin.2009.05.019
Ghosh DC, Bhattacharyya S (2004) Molecular orbital and density functional study of the formation, charge transfer, bonding and the conformational isomerism of the boron trifluoride (BF3) and ammonia (NH3) donor–acceptor complex. Int J Mol Sci 5(8):239–264. https://doi.org/10.3390/i5050239
Harbola MK, Chattaraj PK, Parr RG (1991) Aspects of the softness and hardness concepts of density functional theory. J Am Chem Soc 31(4):395–402. https://doi.org/10.1002/ijch.199100045
Chattaraj PK, Giri S (2007) Stability, reactivity and aromaticity of compounds of a multivalent superatom. J Phys Chem A 111(43):11116–11121. https://doi.org/10.1021/jp0760758
Fujimoto H, Kato S, Yamabe S, Fukui K (1974) Molecular orbital calculations of the electronic structure of borazane. J Chem Phys 60(2):572–578. https://doi.org/10.1063/1.1681075
Chamorro E, Chattaraj PK, Fuentealba P (2003) Variation of the electrophilicity index along the reaction path. J Phys Chem A 107(36):7068–7072. https://doi.org/10.1021/jp035435y
Sarkar U, Chattaraj PK (2021) Reactivity dynamics. J Phys Chem A 125(10):2051–2060. https://doi.org/10.1021/acs.jpca.0c.10788
Patra SG, Mondal H, Chattaraj PK (2022) Variation in electrophilicity on electronic excitation. J Phys Org Chem. https://doi.org/10.1002/poc.4359
Sengupta C, Mitra P, Seth BK, Mandal D, Basu S (2017) Electronic and spatial control over the formation of transient ion pairs during photoinduced electron transfer between proflavin–amine systems in a subpicosecond time regime. RSC Adv 7:15149–15157. https://doi.org/10.1039/C6RA28286E
Seth BK, Sau A, Pal U, Basu B, Chakraborty B (2020) Interaction of proflavin with tryptophan in reverse micellar microenvironment of AOT: photoinduced electron transfer probed by magnetic field effect. J Lumin 220:116953. https://doi.org/10.1016/j.jlumin.2019.116953
Sengupta C, Basu S (2015) A spectroscopic study to decipher the mode of interaction of some common acridine derivatives with CT DNA within nanosecond and femtosecond time domains. RSC Adv 5(95):78160–78171. https://doi.org/10.1039/C5RA13035B
Nenadovic MT, Micic OI, Kosanic MM (1981) Electron transfer reactions from proflavin or acridine yellow radical anions to on acceptors and the possibility of water reduction. Radiat Phys Chem 17(3):159–161. https://doi.org/10.1016/0146-5724(81)90266-1
Chakraborty B, Basu S (2011) deciphering the host-guest chemistry of acridine yellow and cucurbit[7]uril: an integrated spectroscopic and calorimetric study. Chem Phys Lett 507(1–3):74–79. https://doi.org/10.1016/j.cplett.2011.03.014
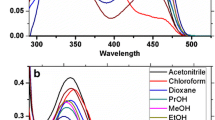
Aaron JJ, Maafi M, Parkanyi C, Boniface C (1995) Quantitative treatment of the solvent effects on the electronic absorption and fluorescence spectra of acridines and phenazines. The ground and first excited singlet-state dipole moments. Spectrochim Acta 51(4):603–615. https://doi.org/10.1016/0584-8539(94)00164-7
Acknowledgements
The authors are thankful to Professor Samita Basu, Saha Institute of Nuclear Physics, India for her valuable suggestions during the preparation of the manuscript.
Funding
There are no funders to report for this submission.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ranjan, P., Chakraborty, B. & Chakraborty, T. A systematic computational study of acridine derivatives through conceptual density functional theory. Mol Divers 27, 1271–1283 (2023). https://doi.org/10.1007/s11030-022-10486-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-022-10486-6