Abstract
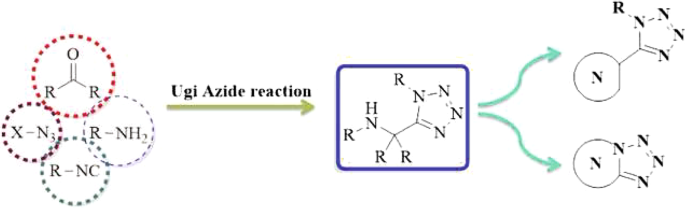
Ugi-azide four-component reaction (UA-4CR) as development on Ugi four-component reaction (U-4CR) is the condensation reaction involving an aldehyde, an amine, an isocyanide, and an azide source. Nowadays, UA-4CR has been employed for the efficient and facile production of 1,5-disubstituted-1H-tetrazoles (1,5-DS-1H-Ts). Interestingly, the combination of 1,5-DS-1H-Ts with suitable post-transformations in a tandem manner results in the construction of various classes of heterocyclic compounds bearing 1,5-DS-1H-T moiety. This review aims to provide the application of diverse post-Ugi-azide reaction in the preparation of different N-heterocyclic compounds bearing 1,5-DS-1H-T such as substituted and fused 1,5-DS-1H-Ts.
Graphic abstract





























Similar content being viewed by others
References
Bladin J (1885) Ueber von dicyanphenylhydrazin abgeleitete verbindungen. Ber Dtsch Chem Ges 18:1544–1551. https://doi.org/10.1002/cber.188501801335
Benson FR (1947) The chemistry of the tetrazoles. Chem Rev 41:1–61. https://doi.org/10.1021/cr60128a001
Maleki A, Sarvary A (2015) Synthesis of tetrazoles via isocyanide-based reactions. RSC Adv 5:60938–60955. https://doi.org/10.1039/C5RA11531K
Baumann M, Baxendale IR, Ley SV, Nikbin N (2011) An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J Org Chem 7:442. https://doi.org/10.3762/bjoc.7.57
Aalla S, Gilla G, Bojja Y, Anumula RR, Vummenthala PR, Padi PR (2012) An efficient and telescopic process for valsartan, an angiotensin II receptor blocker. Org Process Res Dev 16:682–686. https://doi.org/10.1021/op3000306
Yamada T, Kuno A, Masuda K, Ogawa K, Sogawa M, Nakamura S, Ando T, Sano H, Nakazawa T, Ohara H (2003) Candesartan, an angiotensin II receptor antagonist, suppresses pancreatic inflammation and fibrosis in rats. J Pharmacol Exp Ther 307:17–23. https://doi.org/10.1124/jpet.103.053322
Nayak M, Batra S (2010) Isonitriles from the Baylis-Hillman adducts of acrylates: viable precursor to tetrazolo-fused diazepinones via post-Ugi cyclization. Tetrahedron Lett 51:510–516. https://doi.org/10.1016/j.tetlet.2009.11.051
García G, Rodríguez-Puyol M, Rn Alajarín, Serrano I, Sánchez-Alonso P, Griera M, Vaquero JJ, Rodríguez-Puyol D, Álvarez-Builla J, MaL Díez-Marqués (2009) Losartan-antioxidant hybrids: novel molecules for the prevention of hypertension-induced cardiovascular damage. J Med Chem 52:7220–7227. https://doi.org/10.1021/jm9003957
Breschi MC, Calderone V, Digiacomo M, Martelli A, Martinotti E, Minutolo F, Rapposelli S, Balsamo A (2004) NO-sartans: a new class of pharmacodynamic hybrids as cardiovascular drugs. J Med Chem 47:5597–5600. https://doi.org/10.1021/jm049681p
Roh J, Vávrová K, Hrabálek A (2012) Synthesis and functionalization of 5-substituted tetrazoles. Eur J Org Chem 2012:6101–6118. https://doi.org/10.1002/ejoc.201200469
Herr RJ (2002) 5-Substituted-1H-tetrazoles as carboxylic acid isosteres: medicinal chemistry and synthetic methods. Bioorg Med Chem 10:3379–3393. https://doi.org/10.1016/S0968-0896(02)00239-0
Zabrocki J, Smith GD, Dunbar JB, Iijima H, Marshall GR (1988) Conformational mimicry. 1. 1,5-disubstituted tetrazole ring as a surrogate for the cis amide bond. J Chem Soc Chem 110:5875–5880. https://doi.org/10.1021/ja00225a045
Myznikov L, Hrabalek A, Koldobskii G (2007) Drugs in the tetrazole series. Chem Heterocycl Compd 43:1–9. https://doi.org/10.1007/s10593-007-0001-5
Ostrovskii V, Trifonov R, Popova E (2012) Medicinal chemistry of tetrazoles. Russ Chem Bull 61:768–780. https://doi.org/10.1007/s11172-012-0108-4
Bräse S, Gil C, Knepper K, Zimmermann V (2005) Organic azides: an exploding diversity of a unique class of compounds. Angew Chem Int Ed 44:5188–5240. https://doi.org/10.1002/anie.200400657
Scriven EF, Turnbull K (1988) Azides: their preparation and synthetic uses. Chem Rev 88:297–368. https://doi.org/10.1021/cr00084a001
Akritopoulou-Zanze I (2008) Isocyanide-based multicomponent reactions in drug discovery. Curr Opin Chem Biol 12:324–331. https://doi.org/10.1016/j.cbpa.2008.02.004
Dömling A, Ugi I (2000) Multicomponent reactions with isocyanides. Angew Chem Int Ed 39:3168–3210. https://doi.org/10.1002/1521-3773(20000915)39:18%3c3168:AID-ANIE3168%3e3.0.CO;2-U
Dömling A (2006) Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem Rev 106:17–89. https://doi.org/10.1021/cr0505728
Ulaczyk-Lesanko A, Hall DG (2005) Wanted: new multicomponent reactions for generating libraries of polycyclic natural products. Curr Opin Chem Biol 9:266–276. https://doi.org/10.1016/j.cbpa.2005.04.003
Toure BB, Hall DG (2009) Natural product synthesis using multicomponent reaction strategies. Chem Rev 109:4439–4486. https://doi.org/10.1021/cr800296p
Ugi I, Meyr R, Steinbruckner C (1959) Versammlungsberichte. Angew Chem 71:373–388. https://doi.org/10.1002/ange.19590712012
Ugi I, Steinbrückner C (1961) Isonitrile, II. reaktion von isonitrilen mit carbonylverbindungen, aminen und stickstoffwasserstoffsäure. Chem Ber 94:734–742. https://doi.org/10.1002/cber.19610940323
Cano PA, Islas-Jácome A, González-Marrero J, Yépez-Mulia L, Calzada F, Gámez-Montaño R (2014) Synthesis of 3-tetrazolylmethyl-4H-chromen-4-ones via Ugi-azide and biological evaluation against Entamoeba histolytica, Giardia lamblia and Trichomona vaginalis. Bioorg Med Chem 22:1370–1376. https://doi.org/10.1016/j.bmc.2013.12.069
Safa KD, Shokri T, Abbasi H, Teimuri-Mofrad R (2014) One-pot synthesis of new 1,5-disubstituted tetrazoles bearing 2,2-bis(trimethylsilyl)ethenyl groups via the Ugi four-component condensation reaction catalyzed by MgBr 2 2Et2O. J Heterocycl Chem 51:80–84. https://doi.org/10.1002/jhet.1858
Gunawan S, Hulme C (2013) Bifunctional building blocks in the Ugi-azide condensation reaction: a general strategy toward exploration of new molecular diversity. Org Biomol Chem 11:6036–6046. https://doi.org/10.1039/C3OB40900G
Ramezanpour S, Balalaie S, Rominger F, Alavijeh NS, Bijanzadeh HR (2013) Facile, efficient and diastereoselective synthesis of α-hydrazine tetrazoles through a novel one-pot four-component reaction. Tetrahedron 69:10718–10723. https://doi.org/10.1016/j.tet.2013.10.062
Ugi I, Bodesheim F (1961) Isonitrile, VIII. umsetzung von isonitrilen mit hydrazonen und stickstoffwasserstoffsäure. Chem Ber 94:2797–2801. https://doi.org/10.1002/cber.19610941031
Borisov RS, Polyakov AI, Medvedeva LA, Khrustalev VN, Guranova NI, Voskressensky LG (2010) Concise approach toward tetrazolo[1,5-a][1,4]benzodiazepines via a novel multicomponent isocyanide-based condensation. Org Lett 12:3894–3897. https://doi.org/10.1021/ol101590w
El Kaim L, Grimaud L, Purumandla SR (2012) Four-component synthesis of indazole through Ugi-azide coupling. Synlett 2012:295–297. https://doi.org/10.1055/s-0031-1290075
Nixey T, Kelly M, Semin D, Hulme C (2002) Short solution phase preparation of fused azepine-tetrazoles via a UDC (Ugi/de-Boc/cyclize) strategy. Tetrahedron Lett 43:3681–3684. https://doi.org/10.1016/S0040-4039(02)00636-6
Unnamatla MB, Islas-Jácome A, Quezada-Soto A, Ramirez-Lopez SC, Flores-Álamo M, Gamez-Montano R (2016) Multicomponent one-pot synthesis of 3-tetrazolyl and 3-imidazo[1,2-a]pyridin tetrazolo[1,5-a]quinolines. J Org Chem 81:10576–10583. https://doi.org/10.1021/acs.joc.6b01576
Reddy BS, Kota K, Rao BM, Sridhar B, Mukkanti K (2016) Four-component, five-centered, one-pot synthesis of 1-(1H-tetrazol-5-yl)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole derivatives. Tetrahedron Lett 57:4529–4532. https://doi.org/10.1016/j.tetlet.2016.08.067
Heravi MM, Alishiri T (2014) Dimethyl acetylenedicarboxylate as a building block in heterocyclic synthesis. Adv Heterocycl Chem 113:1–66. https://doi.org/10.1016/B978-0-12-800170-7.00001-8
Heravi MM, Talaei B (2014) Ketenes as privileged synthons in the syntheses of heterocyclic compounds. Part 1: Three-and four-membered heterocycles. Adv Heterocycl Chem 113:143–244. https://doi.org/10.1016/B978-0-12-800170-7.00004-3
Heravi MM, Talaei B (2016) Ketenes as privileged synthons in the synthesis of heterocyclic compounds Part 3: Six-membered heterocycles. Adv Heterocycl Chem 118:195–291. https://doi.org/10.1016/bs.aihch.2015.10.007
Heravi MM, Khaghaninejad S, Mostofi M (2014) Pechmann reaction in the synthesis of coumarin derivatives. Adv Heterocycl Chem 112:1–50. https://doi.org/10.1016/B978-0-12-800171-4.00001-9
Heravi MM, Khaghaninejad S, Nazari N (2014) Bischler–Napieralski reaction in the syntheses of isoquinolines. Adv Heterocycl Chem 112:183–234. https://doi.org/10.1016/B978-0-12-800171-4.00005-6
Heravi MM, Talaei B (2015) Ketenes as privileged synthons in the syntheses of heterocyclic compounds part 2: five-membered heterocycles. Adv Heterocycl Chem 114:147–225. https://doi.org/10.1016/bs.aihch.2015.02.001
Heravi MM, Vavsari VF (2015) Recent advances in application of amino acids: key building blocks in design and syntheses of heterocyclic compounds. Adv Heterocycl Chem 114:77–145. https://doi.org/10.1016/bs.aihch.2015.02.002
Heravi MM, Zadsirjan V (2015) Recent advances in the synthesis of benzo[b]furans. Adv Heterocycl Chem 117:261–376. https://doi.org/10.1016/bs.aihch.2015.08.003
Saeedi M, Heravi MM, Beheshtiha YS, Oskooie HA (2010) One-pot three-component synthesis of the spiroacenaphthylene derivatives. Tetrahedron 66:5345–5348. https://doi.org/10.1016/j.tet.2010.05.067
Heravi MM, Sadjadi S, Haj NM, Oskooie HA, Shoar RH, Bamoharram FF (2009) A novel multi-component synthesis of 4-arylaminoquinazolines. Tetrahedron Lett 50:943–945. https://doi.org/10.1016/j.tetlet.2008.12.044
Heravi MM, Mousavizadeh F, Ghobadi N, Tajbakhsh M (2014) A green and convenient protocol for the synthesis of novel pyrazolopyranopyrimidines via a one-pot, four-component reaction in water. Tetrahedron Lett 55:1226–1228. https://doi.org/10.1016/j.tetlet.2014.01.004
Sadjadi S, Heravi MM (2011) Recent application of isocyanides in synthesis of heterocycles. Tetrahedron 67:2707–2752. https://doi.org/10.1016/j.tet.2011.01.086
Heravi MM, Moghimi S (2011) Catalytic multicomponent reactions based on isocyanides. J Iran Chem Soc 8:306–373. https://doi.org/10.1007/BF03249069
Sadjadi S, Heravi MM, Nazari N (2016) Isocyanide-based multicomponent reactions in the synthesis of heterocycles. RSC Adv 6:53203–53272. https://doi.org/10.1039/C6RA02143C
Heravi MM, Mohammadkhani L (2019) Synthesis of various N-heterocycles using the four-component Ugi reaction. Adv Heterocycl Chem. https://doi.org/10.1016/bs.aihch.2019.04.001
Gunawan S, Petit J, Hulme C (2012) Concise one-pot preparation of unique bis-pyrrolidinone tetrazoles. ACS Comb Sci 14:160–163. https://doi.org/10.1021/co200209a
Gunawan S, Keck K, Laetsch A, Hulme C (2012) Synthesis of peptidomimetics, δ- and ε-lactam tetrazoles. Mol Divers 16:601–606. https://doi.org/10.1007/s11030-012-9373-2
Stolyarenko VY, Evdokimov AA, Shishkin VI (2013) Synthesis of tetrazole-substituted spirocyclic γ-lactams by one-pot azido-Ugi reaction–cyclization. Mendeleev Commun 2:108–109. https://doi.org/10.1016/j.mencom.2013.03.020
Medda F, Hulme C (2012) A facile and rapid route for the synthesis of novel 1,5-substituted tetrazole hydantoins and thiohydantoins via a TMSN3-Ugi/RNCX cyclization. Tetrahedron Lett 53:5593–5596. https://doi.org/10.1016/j.tetlet.2012.07.135
Marcos CF, Marcaccini S, Menchi G, Pepino R, Torroba T (2008) Studies on isocyanides: synthesis of tetrazolyl-isoindolinones via tandem Ugi four-component condensation/intramolecular amidation. Tetrahedron Lett 49:149–152. https://doi.org/10.1016/j.tetlet.2007.10.154
Rentería-Gómez A, Islas-Jácome A, Cruz-Jiménez AE, Manzano-Velázquez JC, Rojas-Lima S, Jiménez-Halla JOC, Gámez-Montaño R (2016) Synthesis of 2-tetrazolylmethyl-isoindolin-1-ones via a one-pot Ugi-Azide/(N-acylation/exo-Diels–Alder)/dehydration process. ACS Omega 1:943–951. https://doi.org/10.1021/acsomega.6b00281
Foley C, Shaw A, Hulme C (2016) Two-step route to diverse N-functionalized peptidomimetic-like isatins through an oxidation/intramolecular oxidative-amidation cascade of Ugi azide and ugi three-component reaction products. Org Lett 18:4904–4907. https://doi.org/10.1021/acs.orglett.6b02383
Sharma M, Khan I, Khan S, Mahar R, Shukla SK, Kant R, Chauhan PM (2015) Facile ligand-free Pd-catalyzed tandem C–C/C–N coupling reaction: a novel access to highly diverse tetrazole tag isoindoline derivatives. Tetrahedron Lett 56:5401–5408. https://doi.org/10.1016/j.tetlet.2015.08.008
Wu R, Gao S, Chen X, Yang G, Pan L, Hu G, Jia P, Zhong W, Yu C (2014) Synthesis of 1-(1H-tetrazol-5-yl)-2H-isoindole derivatives through Ugi four-component and silver-catalyzed reactions. Eur J Org Chem 2014:3379–3386. https://doi.org/10.1002/ejoc.201402098
Gunawan S, Ayaz M, De Moliner F, Frett B, Kaiser C, Patrick N, Xu Z, Hulme C (2012) Synthesis of tetrazolo-fused benzodiazepines and benzodiazepinones by a two-step protocol using an Ugi-azide reaction for initial diversity generation. Tetrahedron 68:5606–5611. https://doi.org/10.1016/j.tet.2012.04.068
Cárdenas-Galindo LE, Islas-Jácome A, Alvarez-Rodríguez NV, El Kaim L, Gámez-Montaño R (2014) Synthesis of 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines by a one-pot Ugi-azide/Pictet–Spengler process. Synthesis 46:49–56. https://doi.org/10.1055/s-0033-1340051
Ghandi M, Rahimi S, Zarezadeh N (2017) Synthesis of novel tetrazole containing quinoline and 2,3,4,9-tetrahydro-1H-β-carboline derivatives. J Heterocycl Chem 54:102–109. https://doi.org/10.1002/jhet.2546
Ghandi M, Ahangaran MM, Abbasi A (2017) Sequential one-pot five-component synthesis of tetrazole-based spirotetrahydro-β-carbolines. J Iran Chem Soc 14:1131–1137. https://doi.org/10.1007/s13738-017-1063-7
Gordillo-Cruz RE, Rentería-Gómez A, Islas-Jácome A, Cortes-García CJ, Díaz-Cervantes E, Robles J, Gámez-Montaño R (2013) Synthesis of 3-tetrazolylmethyl-azepino[4,5-b] indol-4-ones in two reaction steps:(Ugi-azide/N-acylation/SN2)/free radical cyclization and docking studies to a 5-Ht 6 model. Org Biomol Chem 11:6470–6476. https://doi.org/10.1039/C3OB41349G
Alvarez-Rodríguez NV, Islas-Jácome A, Rentería-Gómez A, Cárdenas-Galindo LE, Unnamatla MB, Gámez-Montaño R (2018) Synthesis of 1′-tetrazolylmethyl-spiro [pyrrolidine-3, 3′-oxindoles] via two coupled one-pot processes Ugi-azide/Pictet–Spengler and oxidative spiro-rearrangement. New J Chem 42:1600–1603. https://doi.org/10.1039/C7NJ03829A
Bienaymé H, Bouzid K (1998) Synthesis of rigid hydrophobic tetrazoles using an Ugi multi-component heterocyclic condensation. Tetrahedron Lett 39:2735–2738. https://doi.org/10.1016/S0040-4039(98)00283-4
Nixey T, Kelly M, Hulme C (2000) The one-pot solution phase preparation of fused tetrazole-ketopiperazines. Tetrahedron Lett 41:8729–8733. https://doi.org/10.1016/S0040-4039(00)01563-X
Umkehrer M, Kolb J, Burdack C, Ross G, Hiller W (2004) Synthesis of tetrazolopiperazine building blocks by a novel multi-component reaction. Tetrahedron Lett 45:6421–6424. https://doi.org/10.1016/j.tetlet.2004.06.133
Kalinski C, Umkehrer M, Gonnard S, Jäger N, Ross G, Hiller W (2006) A new and versatile Ugi/SNAr synthesis of fused 4,5-dihydrotetrazolo[1,5-a]quinoxalines. Tetrahedron Lett 47:2041–2044. https://doi.org/10.1016/j.tetlet.2006.01.027
Patil P, Kurpiewska K, Kalinowska-Tłuścik J, Dömling A (2017) Ammonia-promoted one-pot tetrazolopiperidinone synthesis by Ugi reaction. ACS Comb Sci 19:343–350. https://doi.org/10.1021/acscombsci.7b00033
Wang Y, Patil P, Kurpiewska K, Kalinowska-Tluscik J, Dömling A (2017) Two cycles with one catch: hydrazine in Ugi 4-CR and its postcyclizations. ACS Comb Sci 19:193–198. https://doi.org/10.1021/acscombsci.7b00009
Yerande SG, Newase KM, Singh B, Boltjes A, Dömling A (2014) Application of cyclic ketones in MCR: Ugi/amide coupling based synthesis of fused tetrazolo[1,5-a][1,4]benzodiazepines. Tetrahedron Lett 55:3263–3266. https://doi.org/10.1016/j.tetlet.2014.04.040
Borisov R, Polyakov A, Medvedeva L, Guranova N, Voskressensky L (2012) Synthesis of tetrazolodiazepines by a five-centered four-component azide Ugi reaction. Scope and limitations. Russ Chem Bull 61:1609–1615. https://doi.org/10.1007/s11172-012-0214-3
Acknowledgements
We are grateful for financial support from the Research Council of Alzahra University. MMH also appreciate the financial support granted by Iran National Science Foundation (INSF) under the given individual research chair.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mohammadkhani, L., Heravi, M.M. Synthesis of N-heterocycles containing 1,5-disubstituted-1H-tetrazole via post-Ugi-azide reaction. Mol Divers 24, 841–853 (2020). https://doi.org/10.1007/s11030-019-09972-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-019-09972-1