Abstract
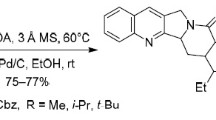
A novel and highly efficient synthetic approach for the expedite construction of new octahydroacridine-isoxazole- and octahydroacridine-1,2,3-triazole-based molecular hybrids is first reported. Rapid access to the octahydroacridine core was achieved in a highly diastereoselective fashion via cationic Povarov reaction of N-propargyl anilines and citronella essential oil (Cymbopogon nardus). The subsequent 1,3-dipolar and Cu (I) catalyzed alkyne-azide cycloaddition reaction of the terminal alkyne fragment with the corresponding oxime or azide affords the desired 3,5-isoxazoles and 1,2,3-triazoles, respectively, as interesting molecular hybrid models for pharmacological studies.




Similar content being viewed by others
References
Beruvé G (2016) An overview of molecular hybrids in drug discovery. Expert Opin Drug Discov 11:281–305. https://doi.org/10.1517/17460441.2016.1135125
Akhtar J, Khan AA, Ali Z, Haider R, Yar MS (2017) Structure-activity relationship (SAR) study and design strategies of nitrogen-containing heterocyclic moieties for their anticancer activities. Eur J Med Chem 125:143–189. https://doi.org/10.1016/j.ejmech.2016.09.023
Xiao ZP, Wang XD, Wang PF, Zhou Y, Zhang JW, Zhang L, Zhou J, Zhou SS, Ouyang H, Lin XY, Mustapa M, Reyinbaike A, Zhum HL (2014) Design, synthesis, and evaluation of novel fluoroquinolone–flavonoid hybrids as potent antibiotics against drug-resistant microorganisms. Eur J Med Chem 80:92–100. https://doi.org/10.1016/j.ejmech.2014.04.037
Ozkay Y, Incesu Z, Onder NI, Tunali Y, Karaca H, Isikdag I, Ucucu Ü (2013) Antimicrobial and anticancer effects of some 2-(substitutedsulfanyl)-N-(5-methyl-isoxazol-3-yl) acetamide derivatives. Med Chem Res 22:211–218. https://doi.org/10.1007/s00044-012-0021-2
Bartlett RR, Schleyerbach R (1985) Immunopharmacological profile of a novel isoxazol derivative, HWA 486, with potential antirheumatic activity–I. Disease modifying action on adjuvant arthritis of the rat. Int J Immunopharmacol 7:7–18. https://doi.org/10.1016/0192-0561(85)90003-7
Murata M, Hasegawa K, Kanazawa I, Japan Zonisamide on PD Study Group (2007) Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology 68:45–50. https://doi.org/10.1212/01.wnl.0000250236.75053.16
Sahu JK, Ganguly S, Kaushik A (2013) Triazoles: a valuable insight into recent developments and biological activities. Chin J Nat Med 11:456–465. https://doi.org/10.1016/S1875-5364(13)60084-9
Müller-Schiffmann A, Sticht H, Korth C (2012) Hybrid compounds: from simple combinations to nanomachines. BioDrugs 26:21–31. https://doi.org/10.2165/11597630-000000000-00000
Mao J, Yuan H, Wang Y, Wan B, Pak D, He R, Franzblau SG (2010) Synthesis and antituberculosis activity of novel mefloquine-isoxazole carboxylic esters as prodrugs. Bioorg Med Chem 20:1263–1268. https://doi.org/10.1016/j.bmcl.2009.11.105
Shekhar AC, Lingaiah BPV, Rao PS, Narsaiah B, Allanki AD, Sijwali PS (2015) Design, synthesis and biological evaluation of novel fluorinated heterocyclic hybrid molecules based on triazole & quinoxaline scaffolds lead to highly potent antimalarials and antibacterials. Lett Drug Des Discov 12:393–407. https://doi.org/10.2174/1570180812666141111235301
Lokanatha Rai KM, Linganna N, Hassner A, Anjanamurthy C (1992) A convenient method for the generation of nitrile oxide and its application to the synthesis of 2-isoxazolines. Org Prep Proc Int 24:91–93. https://doi.org/10.1080/00304949209356711
Kim JN, Ryu EK (1990) A convenient synthesis of nitrile oxides from aldoximes by 1-chlorobenzotriazole. Synth Commun 20:1373–1377. https://doi.org/10.1080/00397919008052851
Hassner A, Lokanatha Rai KM (1989) A new method for the generation of nitrile oxides and its application to the synthesis of 2-isoxazolines. Synthesis 1:57–59. https://doi.org/10.1055/s-1989-27152
Grundmann C, Dean JM (1965) Nitrile oxides. V. Stable aromatic nitrile oxides. J Org Chem 30:2809–2812. https://doi.org/10.1021/jo01019a074
Himo F, Lovell T, Hilgraf R, Rostovtsev VV, Noodleman L, Sharpless KB, Fokin VV (2005) Copper (I)-catalized synthesis of azoles. DFT study predicts unprecedent reactivity and intermediates. J Am Chem Soc 127:210–216. https://doi.org/10.1021/ja0471525
Hein JE, Fokin VV (2010) Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: new reactivity of Copper (I) acetylides. Chem Soc Rev 39:1302–1315. https://doi.org/10.1039/b904091a
Del Giudice MR, Borioni A, Mustazza C, Gatta F (1997) Synthesis of 9-amino-, 9-aminomethyl-1,2,3,4-tetrahydro-and 1,2,3,4,5,6,7,8-octahydroacridine derivatives. J Heterocyclic Chem 34:1661–1667. https://doi.org/10.1002/jhet.5570340604
Ermolaeva VG, Yashunskii VG, Polezhaeva AI, Mashkovskii MD (1968) Synthesis and pharmacological properties of certain derivatives of octahydroacridines. Pharm Chem J 2:310–312. https://doi.org/10.1007/BF00759725
Canas-Rodriguez A, Canas RG, Mateo-Bernardo A (1987) Tricyclic inhibitors of gastric acid secretion. Part V. Octahydroacridines. An Quim Ser C 83:24–27
Mayekar NV, Nayak SK, Chattopadhyay S (2004) Two convenient one-pot strategies for the synthesis of octahydroacridines. Synth Commun 34:3111–3119. https://doi.org/10.1081/SCC-200028567
Laschat S, Noe R, Riedel M (1993) Novel (Imino-η6-arene)chromium complexes and their diastereoselective intramolecular hetero-Diels-Alder reactions. Organometallics 12:3738–3742. https://doi.org/10.18419/opus-6985
Schulte JL, Laschat S, Kotila S, Hecht J, Frölich R, Wibbeling B (1996) Synthesis of η6-(octahydroacridine)-chromiumtricarbonyl complexes with nonpolar tails via molecular sieves-catalized cyclization of N-arylimines and subsequent diastereoselective complexation. Heterocycles 43:2713–2724. https://doi.org/10.3987/COM-96-7622
Acelas M, Romero Bohórquez AR, Kouznetsov VV (2017) Highly diastereoselective synthesis of new trans-fused octahydroacridines via intramolecular cationic imino Diels-Alder reaction of N-protected anilines and citronellal or citronella essential oil. Synthesis 49:2153–2162. https://doi.org/10.1055/s-0036-1588713
Rodríguez YA, Gutiérrez M, Ramírez D, Alzate-Morales J, Bernal CC, Güiza FM, Romero Bohórquez AR (2016) Novel N-allyl/propargyl tetrahydroquinolines: synthesis via three-component cationic imino Diels-Alder reaction, binding prediction and evaluation as cholinesterase inhibitors. Chem Biol Drug Des 88:498–510. https://doi.org/10.1111/cbdd.12773
Van Berkel GJ, McLuckey SA, Glish GL (1992) Electrochemical origin of radical cations observed in electrospray ionization mass spectra. Anal Chem 64:1586–1593. https://doi.org/10.1021/ac00038a015
Crotti AEM, Vessecchi R, Callegari JLC, Lopes NP (2006) Electrospray ionization mass spectrometry: chemical processes involved in the ion formation from low molecular weight organic compounds. Quím Nova 29:287–292. https://doi.org/10.1590/S0100-40422006000200020
Zenobi R, Knochenmuss R (1998) Ion formation in MALDI mass spectrometry. Mass Spectrom Rev 17:337–366. https://doi.org/10.1002/(SICI)1098-2787(1998)17:5%3c337:AID-MAS2%3e3.0.CO;2-S
Stephen O, Nwaukwa MK, Philip MK (1989) Ring chlorination of benzenoid compounds using calcium hypochlorite [Ca(OCl)2]. Synth Commun 19:799–804. https://doi.org/10.1080/00397918908050996
Easton CJ, Hughes CM, Tiekink ERT, Lubin CE, Savage GP, Simpson GW (1994) Reversal of regiochemistry in the synthesis of isoxazoles by nitrile oxide cycloadditions. Tetrahedron Lett 35:3589–3592. https://doi.org/10.1016/S0040-4039(00)73248-5
Colombano G, Albani C, Ottonello G, Ribeiro A, Scarpelli R, Tarozzo G, Daglian J, Jung KM, Piomelli D, Bandiera T (2015) O-(triazolyl)methyl carbamates as a novel and potent class of fatty acid amide hydrolase (FAAH) inhibitors. Chem Med Chem 10:380–395. https://doi.org/10.1002/cmdc.201402374
Shao C, Wang X, Zhang Q, Luo S, Zhao J, Hu Y (2011) Acid-base jointly promoted copper(I)-catalized azide-alkyne cycloaddition. J Org Chem 76:6832–6836. https://doi.org/10.1021/jo200869a
Urankar D, Steinbücher M, Kosjek J, Kosmrlj J (2010) N-(propargyl)diazenecarboxamides for ‘click’ conjugation and their 1,3-dipolar cycloadditions with azidoalkylamines in the presence of Cu(II). Tetrahedron 66:2602–2613. https://doi.org/10.1016/j.tet.2010.02.042
Jwad RS, Mohammed AI, Shihab MS (2012) Synthesis of 1,2,3-triazoles based on phenacyl azide derivatives via click chemistry. Iraqi J Sci 53:487–494
Acknowledgements
This work was supported by Patrimonio Autónomo Fondo Nacional de Financiamiento para la Ciencia, la Tecnología y la Innovación, Francisco José de Caldas (Contract RC-0572-2012). We want to thank Laboratorio de Espectrometria de Masas—Parque Tecnológico Guatiguará for data collection. We also wish to acknowledge Prof. Elena E. Stashenko, Research Director CROM-MASS–CENIVAM, Industrial University of Santander, Colombia, for the generous donation and characterization of the EO of citronella.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Acelas, M., Kouznetsov, V.V. & Romero Bohórquez, A.R. Facile and highly diastereo and regioselective synthesis of novel octahydroacridine-isoxazole and octahydroacridine-1,2,3-triazole molecular hybrids from citronella essential oil. Mol Divers 23, 183–193 (2019). https://doi.org/10.1007/s11030-018-9863-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9863-y