Abstract
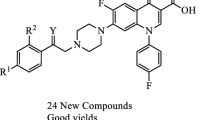
A series of 7-piperazinylquinolones containing a (benzo[d]imidazol-2-yl)methyl moiety were designed and synthesized as new antibacterial agents. The antibacterial activity of title compounds was evaluated against Gram-positive (Staphylococcus aureus, Staphylococcus epidermidis and Bacillus subtilis) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumonia) microorganisms. Among the tested compounds, the N1-cyclopropyl derivative 4a showed the highest activity against S. aureus, S. epidermidis, B. subtilis and E. coli (\(\text {MIC} = 0.097\) \(\upmu \)g/mL), being 2–4 times more potent than reference drug norfloxacin. A structure-activity relationship study demonstrated that the effect of the nitro group on the benzimidazole ring depends on the pattern of substitutions on the piperazinylquinolone.






Similar content being viewed by others
References
Castro W, Navarro M, Biot C (2013) Medicinal potential of ciprofloxacin and its derivatives. Future Med Chem 5:81–96. https://doi.org/10.4155/fmc.12.181
Willmott CJ, Critchlow SE, Eperon IC, Maxwell A (1994) The complex of DNA gyrase and quinolone drugs with DNA forms a barrier to transcription by RNA polymerase. J Mol Biol 242:351–363. https://doi.org/10.1006/jmbi.1994.1586
Drlica K, Zhao X (1997) DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev 61:377–392
Chen AY, Liu LF (1994) DNA topoisomerases: essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol 34:191–218. https://doi.org/10.1146/annurev.pa.34.040194.001203
Gootz TD, Barrett JF, Sutcliffe JA (1990) Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems. Antimicrob Agents Chemother 34:8–12. https://doi.org/10.1128/AAC.34.1.8
Emami S, Shafiee A, Foroumadi A (2005) Quinolones: recent structural and clinical developments. Iran J Pharm Res 3:123–136
Koga H, Itoh A, Murayama S, Suzue S, Irikura T (1980) Structure-activity relationships of antibacterial 6,7- and 7,8-disubstituted 1-alkyl-1,4-dihydro-4-oxoquinoline-3-carboxylic acids. J Med Chem 23:1358–1363. https://doi.org/10.1021/jm00186a014
Emami S, Shafiee A, Foroumadi A (2006) Structural features of new quinolones and relationship to antibacterial activity against Gram-positive bacteria. Mini-Rev Med Chem 6:375–386. https://doi.org/10.2174/138955706776361493
Piddock LJV (1999) Mechanisms of fluoroquinolone resistance: an update 1994–1998. Drugs 58:11–18. https://doi.org/10.2165/00003495-199958002-00003
Appelbaum PC, Hunter PA (2000) The fluoroquinolone antibacterials: past, present and future perspectives. Int J Antimicrob Agents 16:5–15. https://doi.org/10.1016/S0924-8579(00)00192-8
Emami S (2010) New quinolones with potential anti-MRSA activity. Nova Science Publishers Inc, New York
Nakaminami H, Sato-Nakaminami K, Noguchi N (2014) A novel GyrB mutation in meticillin-resistant Staphylococcus aureus (MRSA) confers a high level of resistance to third-generation quinolones. Int J Antimicrob Agents 43:478–479. https://doi.org/10.1016/j.ijantimicag.2014.02.002
Wagman AS, Wentland MP (2007) Quinolone antibacterial agents. In: Taylor JB, Triggle DJ (eds) Comprehensive medicinal chemistry II, vol 7. Elsevier LTD, Oxford, pp 567–596
Seenaiah D, Reddy PR, Reddy GM, Padmaja A, Padmavathi V, Krishna NS (2014) Synthesis, antimicrobial and cytotoxic activities of pyrimidinyl benzoxazole, benzothiazole and benzimidazole. Eur J Med Chem 77:1–7. https://doi.org/10.1016/j.ejmech.2014.02.050
Hosamani KM, Seetharamareddy HR, Keri RS, Hanamanthagouda MS, Moloney MG (2009) Microwave assisted, one-pot synthesis of 5-nitro- 2-aryl substituted-1H-benzimidazole libraries: screening in vitro for antimicrobial activity. J Enzyme Inhib Med Chem 24:1095–1100. https://doi.org/10.1080/14756360802632716
Zhang HZ, Damu GL, Cai GX, Zhou CH (2013) Design, synthesis and antimicrobial evaluation of novel benzimidazole type of Fluconazole analogues and their synergistic effects with Chloromycin, Norfloxacin and Fluconazole. Eur J Med Chem 64:329–344. https://doi.org/10.1016/j.ejmech.2013.03.049
Foroumadi A, Emami S, Hassanzadeh A, Rajaee M, Sokhanvar K, Moshafi MH, Shafiee A (2005) Synthesis and antibacterial activity of N-(5-benzylthio-1,3,4-thiadiazol-2-yl) and N-(5-benzylsulfonyl-1,3,4-thiadiazol-2-yl)piperazinyl quinolone derivatives. Bioorg Med Chem Lett 15:4488–4492. https://doi.org/10.1016/j.bmcl.2005.07.016
Foroumadi A, Firoozpour L, Emami S, Mansouri S, Ebrahimabadi AH, Asadipour A, Amini M, Saeid-Adeli N, Shafiee A (2007) Synthesis and antibacterial activity of N-[5-(chlorobenzylthio)-1,3,4-thiadiazol-2-yl] piperazinyl quinolone derivatives. Arch Pharm Res 30:138–145. https://doi.org/10.1007/BF02977685
Foroumadi A, Firoozpour L, Nematollahi N, Ebrahimabadi AH, Emami S, Moshafi MH, Asadipour A, Shafiee A (2007) Synthesis of new fluoroquinolones containing a N-[5-(fluorobenzylthio)-1,3,4-thiadiazol-2-yl]piperazine moiety. Asian J Chem 19:4547–4552
Foroumadi A, Emami S, Mehni M, Moshafi MH, Shafiee A (2005) Synthesis and antibacterial activity of N-[2-(5-bromothiophen-2-yl)-2-oxoethyl] and N-[(2–5-bromothiophen-2-yl)-2-oximinoethyl] derivatives of piperazinyl quinolones. Bioorg Med Chem Lett 15:4536–4539. https://doi.org/10.1016/j.bmcl.2005.07.005
Foroumadi A, Oboudiat M, Emami S, Karimollah A, Saghaee L, Moshafi MH, Shafiee A (2006) Synthesis and antibacterial activity of N-[2-[5-(methylthio)thiophen-2-yl]-2-oxoethyl] and N-[2-[5-(methylthio)thiophen-2-yl]-2-(oxyimino)ethyl]piperazinylquinolone derivatives. Bioorg Med Chem 14:3421–3427. https://doi.org/10.1016/j.bmc.2005.12.058
Letafat B, Emami S, Mohammadhosseini N, Faramarzi MA, Samadi N, Shafiee A, Foroumadi A (2007) Synthesis and antibacterial activity of new N-[2-(thiophen-3-yl)ethyl] piperazinyl quinolones. Chem Pharm Bull (Tokyo) 55:894–898. https://doi.org/10.1248/cpb.55.894
Mohammadhosseini N, Alipanahi Z, Alipour E, Emami S, Faramarzi MA, Samadi N, Khoshnevis N, Shafiee A, Foroumadi A (2012) Synthesis and antibacterial activity of novel levofloxacin derivatives containing a substituted thienylethyl moiety. Daru J Pharm Sci 20:16; https://doi.org/10.1186/2008-2231-20-16
Shafiee A, Haddad Zahmatkesh M, Mohammadhosseini N, Khalafy J, Emami S, Moshafi MH, Sorkhi M, Foroumadi A (2008) Synthesis and in-vitro antibacterial activity of N-piperazinyl quinolone derivatives with 5-chloro-2-thienyl group. Daru 16:189–195
Foroumadi A, Mohammadhosseini N, Emami S, Letafat B, Faramarzi MA, Samadi N, Shafiee A (2007) Synthesis and antibacterial activity of new 7-piperazinyl-quinolones containing a functionalized 2-(furan-3-yl)ethyl moiety. Arch Pharm 340:47–52. https://doi.org/10.1002/ardp.200600169
Emami S, Foroumadi A, Faramarzi MA, Samadi N (2008) Synthesis and antibacterial activity of quinolone-based compounds containing a coumarin moiety. Arch Pharm 341:42–48. https://doi.org/10.1002/ardp.200700090
Emami S, Foroumadi A, Samadi N, Faramarzi MA, Rajabalian S (2009) Conformationally constrained analogs of N-substituted piperazinylquinolones: synthesis and antibacterial activity of N-(2,3-dihydro-4-hydroxyimino-4H-1-benzopyran-3-yl)-piperazinylquinolones. Arch Pharm 342:405–411. https://doi.org/10.1002/ardp.200800182
Emami S, Ghafouri E, Faramarzi MA, Samadi N, Irannejad H, Foroumadi A (2013) Mannich bases of 7-piperazinylquinolones and kojic acid derivatives: synthesis, in vitro antibacterial activity and in silico study. Eur J Med Chem 68:185–191. https://doi.org/10.1016/j.ejmech.2013.07.032
Baron EJ, Finegold SM (2002) Bailey Scott’s diagnostic microbiology, 11th edn. The C. V. Mosby Company, St. Louis, pp 235–236
Li Q, Xing J, Cheng H, Wang H, Wang J, Wang S, Zhou J, Zhang H (2015) Design, synthesis, antibacterial evaluation and docking study of novel 2-hydroxy-3-(nitroimidazolyl)-propyl-derived quinolone. Chem Biol Drug Des 85:79–90. https://doi.org/10.1111/cbdd.12395
Sheng C, Che X, Wang W, Wang S, Cao Y, Yao J, Miao Z, Zhang W (2011) Design and synthesis of antifungal benzoheterocyclic derivatives by scaffold hopping. Eur J Med Chem 46:1706–1712. https://doi.org/10.1016/j.ejmech.2011.01.075
Foroumadi A, Ashraf-Askari R, Moshafi MH, Emami S, Zeynali A (2003) Synthesis and in vitro antibacterial activity of N-[5-(5-nitro-2-furyl)-1,3,4-thiadiazole-2-yl] piperazinyl quinolone derivatives. Pharmazie 58:432–433
Foroumadi A, Mansouri S, Emami S, Mirzaei J, Sorkhi M, Saeid-Adeli N, Shafiee A (2006) Synthesis and antibacterial activity of nitroaryl thiadiazole-levofloxacin hybrids. Arch Pharm 339:621–624. https://doi.org/10.1002/ardp.200600108
Jazayeri S, Moshafi MH, Firoozpour L, Emami S, Rajabalian S, Haddad M, Pahlavanzadeh F, Esnaashari M, Shafiee A, Foroumadi A (2009) Synthesis and antibacterial activity of nitroaryl thiadiazole-gatifloxacin hybrids. Eur J Med Chem 44:1205–1209. https://doi.org/10.1016/j.ejmech.2008.09.012
Emami S, Shahrokhirad N, Foroumadi A, Faramarzi MA, Samadi N, Soltani-Ghofrani N (2013) 7-Piperazinylquinolones with methylene-bridged nitrofuran scaffold as new antibacterial agents. Med Chem Res 22:5940–5947. https://doi.org/10.1007/s00044-013-0581-9
Acknowledgements
This work was supported by a Grant (No. 833) from the Research Council of Mazandaran University of Medical Sciences, Sari, Iran.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arab, HA., Faramarzi, M.A., Samadi, N. et al. New 7-piperazinylquinolones containing (benzo[d]imidazol-2-yl)methyl moiety as potent antibacterial agents. Mol Divers 22, 815–825 (2018). https://doi.org/10.1007/s11030-018-9834-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9834-3