Abstract
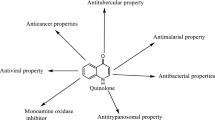
A series ofN-[5-(chlorobenzylthio)-1,3,4-thiadiazol-2-yl] piperazinyl quinolone derivatives (4a-1) have been synthesized by reaction of piperazinyl quinolones with 5-chloro-2-(chloroben-zylthio)-1,3,4-thiadiazoles. Their structures were confirmed by elemental analysis, IR and NMR spectra. The antibacterial activities of4a-1 against a variety of Gram-positive and Gram-negative bacteria were determined. Several compounds showed a good antibacterial activity against Gram-positive bacteria among which, compound 4e with a 2-chlorobenzylthio moiety in ciprofloxacin derivative, exhibited high activities againstStaphylococcus aureus andStaphylococcus epidermidis (MIC=0.06 μg/mL). The structure-activity relationship (SAR) study revealed that the position of chlorine atom on benzyl moiety would dramatically affect the antibacterial activities of the synthesized compounds.
Similar content being viewed by others
References
Alemagna, A., Bachetti, T., and Beltrame, P., 1,3,4-thiadiazoles-IIINucleophilic reactivity of 2-aryl-5-chloro derivatives.Tetrahedron, 24, 3209–3217 (1968).
Anderson, V. E. and Osherhoff, N., Type II topoisomerases astargets for quinolone antibacterials: turning Dr. Jekyll into Mr.Hyde.Curr. Pharm. Des., 7, 337–353 (2001).
Baron, E. J. and Finegold, S. M., Bailey, Scott’s DiagnosticMicrobiology, 8th edition, C. V. Mosby: St. Louis, (1990).
Chu, D. T., Fernandes, P. B., Claiborne, A. K., Pihuleac, E.,Nordeen, C. W., Maleczka, R. E., and Pernet, A. G, Synthesisand structure-activity relationships of novel arylfluoroquinoloneantibacterial agents.J. Med. Chem., 28, 1558–64 (1985).
De Sarro, A. and De Sarro, G, Adverse reactions to fluoro-quinolones.An overview on mechanistic aspects.Curr. Med. Chem., 8, 371–374(2001).
Domagala, J. M., Heifetz, C. L, Hutt, M. P., Mich, T. F, Nichols,J. B., Solomon, M., and Worth, D. F., 1-Substituted 7-[3-[(ethylamino)methyl]-1-pyrrolidinyl]-6,8-difluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acids.New quantitative structure-activity relationships at N1 for the quinolone antibacterials.J.Med. Chem., 31, 991–1001 (1988).
Emami, S., Shafiee, A., and Foroumadi, A., Structural featuresof new quinolones and relationship to antibacterial activityagainst gram-positive bacteria.Mini-Rev. Med. Chem., 6,375–386 (2006).
Fang, K. C, Chen, Y. L, Sheu, J. Y, Wang, T. C, and Tzeng, C.C, Synthesis, antibacterial, and cytotoxic evaluation ofcertain 7-substituted norfloxacin derivatives.J. Med. Chem.,43,3809–3812(2000).
Foroumadi, A., Emami, S., Mehni, M., Moshafi, M. H., andShafiee, A., Synthesis and antibacterial activity of N-[2-(5-bromothiophen-2-yl)-2-oxoethyl]and N-[2-(5-bromothiophen-2-yl)-2-oximinoethyl] derivatives of piperazinyl quinolones.Bioorg. Med. Chem. Lett., 20,4536–4539 (2005a).
Foroumadi, A., Emami, S., Hassanzadeh, A., Rajaee, M.,Sokhanvar, K., Moshafi, M. H., and Shafiee, A., SynthesisAnd antibacterial activity of N-(5-benzylthio-1,3,4-thiadiazol-2-yl) andN-(5-benzylsulfonyl-1,3,4-thiadiazol-2-yl)piperazinylquinolone derivatives.Bioorg. Med. Chem. Lett., 15, 4488–4492 (2005b).
Gootz, T. D., McGuirk, P. R., Moynihan, M. S., and Haskell, S.L, Placement of alkyl substituents on the C-7 piperazine ringof fluoroquinolones: dramatic differential effects on mammaliantopoisomerase II and DNA gyrase.Antimicrob.Agents. Chemother., 38,130–133 (1994).
Hagihara, M., Kashiwase, H., Katsube, T., Kimura, T., Komai, T.,Momota, K., Ohmine, T., Nishigaki, T., Kimura, S., andShimada, K., Synthesis and anti-HIV activity of arylpiperazinylfluoroquinolones: a new class of anti-HIV agents.Bioorg.Med. Chem. Lett., 9, 3063–3068 (1999).
Pandeya, S. N., Sriram, D., Nath, G, and De Clercq, E.,Synthesis, antibacterial, antifungal and anti-HIV activities ofnorfloxacin mannich bases.Eur. J. Med. Chem., 35, 249–255(2000).
Shafiee, A. and Foroumadi, A., Synthesis of arylsulfonyl-1,3,4-thiadiazoles.J. Heterocycl. Chem., 33,1611–1614 (1996).
Shen, L. L, Mitscher, L. A., Sharma, P. N., O’Donnell, T. J., Chu,D. W., Cooper, C. S., Rosen, T., and Pernet, A. G, Mechanismof inhibition of DNA gyrase by quinolone antibacterials: acooperative drug-DNA binding model.Biochemistry, 28,3886–3894(1989).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Foroumadi, A., Firoozpour, L., Emami, S. et al. Synthesis and antibacterial activity of N-[5-chlorobenzylthio-1,3,4-thiadiazol-2-yl] piperazinyl quinolone derivatives. Arch Pharm Res 30, 138–145 (2007). https://doi.org/10.1007/BF02977685
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977685