Abstract
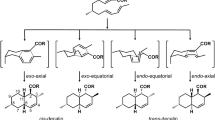
Functionalizations of cycloadducts are important steps for the use of Diels–Alder reactions in the construction of complex cyclic or polycyclic molecules from relatively simple starting materials. In the present work, we studied the ability of Penicillium brasilianum to perform microbial transformations of racemic Diels–Alder endo-cycloadducts. Thus, Diels–Alder products, obtained from reacting cyclopentadiene or 2,3-dimethylbutadiene with alkylated para-benzoquinones, were transformed by the resting cells of P. brasilianum producing new functionalized polycyclic compounds. These biotransformations yielded novel products of oxidation and ring closure, reduction of the C=C or C=O in \(\upalpha , \upbeta \)-unsaturated system, and allylic hydroxylations. The reduction products (conjugated double bond and carbonyl group) were also synthesized, and the enantioselectivity of both in vitro and in vivo processes was evaluated. In all cases, the microbiological transformations were enantioselective. In silico docking studies of the Diels–Alder cycloadducts with P. brasilianum oxidoreductase “old yellow enzymes” shed more light on these transformations.






Similar content being viewed by others
References
Brocksom TJ, Donatoni MC, Uliana MP, Vieira YW (2010) A reação de Diels-Alder no início do século vinte um. Quim Nova 33:211–2218. doi:10.1590/S0100-40422010001000034
Marcos IS, Castañeda L, Basabe P, Díez D, Urones JG (2012) Labdane diterpenes with highly functionalized B rings. Mini-Rev Org Chem 9:54–86. doi:10.2174/157019312799080044
Peters RJ (2010) Two rings in them all: The labdane-related diterpenoids. Nat Prod Rep 27:1521–1530. doi:10.1039/c0np00019a
Schubert M, Metz P (2011) Enantioselective total synthesis of the diterpenes kempene-2, kempene-1, and 3-epi-kempene-1 from the defense secretion of higher termites. Angew Chem Int Ed 50:2954–2956. doi:10.1002/anie.201007551
Ito FM, Petroni JM, Lima DP, Beatriz A, Marques MR, Moraes MO, Costa-Lotufo LV, Montenegro RC, Magalhães HIF, Pessoa C (2007) Synthesis and biological evaluation of rigid polycyclic derivatives of the Diels-Alder adduct tricyclo[6.2.1.02,7]undeca-4,9-dien-3,6-dione. Molecules 12:271–282. doi:10.3390/12020271
Geldenhuys WJ, Malan SF, Bloomquist JR, Marchand AP, Schyf C (2005) Pharmacology and structure-activity relationships of bioactive polycyclic cage compounds: A focus on pentacycloundecane derivatives. Med Res Rev 25:21–48. doi:10.1002/med.20013
Clouthier CM, Pelletier JN (2012) Expanding the organic toolbox: a guide to integrating biocatalysis in synthesis. Chem Soc Rev 41:1585–1605. doi:10.1039/C2CS15286J
Ito FM, Mena AEM, Marques MR, Lima DP, Beatriz A (2009) Biotransformation of a cage-like Diels-Alder adduct and derivatives by Mucor ramosissimus Samutsevitsch. Braz J Microbiol 40:563–568. doi:10.1590/S1517-83822009000300019
Fill TP, da Silva JV, OLiveira KT, da Silva BF, Rodrigues-Filho E (2012) Oxidative potential of some endophytic fungi using 1-indanone as a substrate. J Microb Biot 22:832–837. doi:10.4014/jmb.1112.12014
Donatoni MC, Junior GAB, Oliveira KT, Ando RA, Brocksom TJ, dos Santos AA (2014) Solvent-free DielseAlder reactions catalyzed by \(\text{ FeCl }_{3}\) on aerosil silica. Tetrahedron 70:3231–3238. doi:10.1016/j.tet.2014.02.017
Panagiotou G, Olavarria R, Olsson L (2007) Penicillium brasilianum as an enzyme factory; the essential role of feruloyl esterases for the hydrolysis of the plant cell wall. J Biot 130:219–228. doi:10.1016/j.jbiotec.2007.04.011
Fill TP, Asenha HB, Marques AS, Ferreira AG, Rodrigues-Filho E (2013) Time course production of indole alkaloids by an endophytic strain of Penicillium brasilianum cultivated in rice. Nat Prod Res 27:967–974. doi:10.1080/14786419.2012.701210
Fill TP, da Silva BF, Rodrigues-Filho E (2010) Biosynthesis of Phenylpropanoid amides by an endophytic Penicillium brasilianum found in root bark of Melia azedarach. J Microb Biot 20:622–629. doi:10.4014/jmb.0908.08018
dos Santos RMG, Rodrigues-Filho E (2003) Structures of meroterpenes produced by Penicillium sp, an endophytic fungus found associated with Melia azedarach. J Braz Chem Soc 14:722–727. doi:10.1590/S0103-50532003000500004
Fill TP, Pereira GK, dos Santos RMG, Rodrigues-Filho E (2007) Four additional meroterpenes produced by Penicillium sp found in association with Melia azedarach. Possible biosynthetic intermediates to austin. Z Naturforsch B 62:1035–1044. doi:10.1515/znb-2007-0806
Marchand AP, Dong EZ, Bott SG (1998) Synthesis and acid- and base-promoted ring opening of polycarbocyclic oxiranes. Tetrahedron 54:4459–4470. doi:10.1016/S0040-4020(98)00162-8
Toogood HS, Gardiner JM, Scrutton NS (2010) Biocatalytic reductions and chemical versatility of the old yellow enzyme family of flavoprotein oxidoreductases. ChemCatChem 2:892–914. doi:10.1002/cctc.201000094
Steinkellner G, Gruber CC, Pavkov-Keller T, Steiner ABK, Łyskowski CWA, Schwamberger O, Oberer M, Schwab H, Faber K, Macheroux P, Gruber K (2014) Identification of promiscuous ene-reductase activity by mining structural databases using active site constellations. Nat Commun 5:1–9. doi:10.1038/ncomms5150
Johansson MU, Zoete V, Michielin O, Guex N (2012) Defining and searching for structural motifs using DeepView/Swiss-PdbViewer. BMC Bioinformatics 13:1–10. doi:10.1186/1471-2105-13-173
Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J (2006) CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res 34:116–118. doi:10.1093/nar/gkl282
dos Santos RMG, Rodrigues-Filho E, Rocha WC, Teixeira MFS (2003) Endophytic fungi from Melia azedarach. World J Microb Biot 19:767–770. doi:10.1023/A:1026000731189
Andrienko GA (2015) Chemcraft basics, http://www.chemcraftprog.com (accessed October, 2015)
Hanwell MD, Curtis DE, Lonie DC, Vandermeerschd T, Zurek E, Hutchison GR (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminformatics 4:1–17. doi:10.1186/1758-2946-4-17
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. doi:10.1002/jcc.21256
Acknowledgments
The authors are grateful to the Brazilian institutions FAPESP—Fundação de Amparo à Pesquisa do Estado de São Paulo (2010/11384-6 and 2011/13993-2), CNPq—Conselho Nacional de Desenvolvimento Científico e Tecnológico, and CAPES—Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior for the financial support and fellowships.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Din, Z.U., Fill, T.P., Donatoni, M.C. et al. Microbial diversification of Diels–Alder cycloadducts by whole cells of Penicillium brasilianum . Mol Divers 20, 877–885 (2016). https://doi.org/10.1007/s11030-016-9680-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9680-0