Abstract
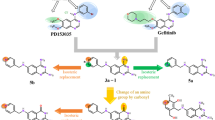
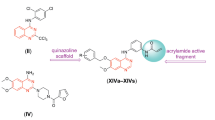
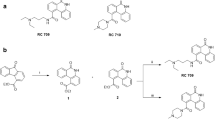
Quinazoline-2,4(\(1H,3H\))-diones exhibit a wealth of biological activities including antitumor proliferation. We established an improved method for the synthesis of quinazoline-2,4(\(1H,3H\))-dione derivatives with three points of molecular diversity. Data indicate that compounds 60 (average \(\text{ logGI}_{50} \!=\! -6.1\)), 65 (average \(\text{ logGI}_{50} \!=\! -6.13\)), 69 (average \(\text{ logGI}_{50} \!=\! -6.44\)), 72 (average \(\text{ logGI}_{50} \!=\! -6.39\)), and 86 (average \(\text{ logGI}_{50} = -6.45\)) significantly inhibited the in vitro growth of 60 human tumor cell lines tested. Structure–activity relationship analyses indicate that chlorophenethylureido is the necessary substituent at the \(\text{ D}_{3}\) diversity point (7-position of quinazoline-2,4(\(1H,3H\))-dione), in particular, \(o\)-chlorophenethylurea (69) achieved optimal activity. \(o\)- or \(m\)-Chlorophenethyl substitutions (69 and 72) at the \(\text{ D}_{2}\) diversity point (3-position of quinazo line-2,4(\(1H,3H\))-dione) gave the most potent compounds. Methoxyl and 4-methylpiperazin-1-yl substitution at the \(\text{ D}_{1}\) diversity point (6-position of quinazoline-2,4(\(1H,3H\))-dione skeleton) may yield better activity than other groups. The quinazoline-2,4(\(1H,3H\))-dione scaffold can be effectively replaced by 2\(H\)-benzo[b][1,4]thiazin-3(4\(H\))-one.










Similar content being viewed by others
References
Michael DH, Michael AC, Margaret Z, Debra LH, Laura AS, Mark CS, Glenn WG, Jeffrey WG, Edmund E, Michael AS, Stephen JG (2007) In vitro and in vivo activities of PD 0305970 and PD 0326448, new bacterial gyrase/topoisomerase inhibitors with potent antibacterial activities versus multidrug-resistant gram-Ppositive and fastidious organism groups. Antimicrob Agents Chemother 51:1191–1201. doi:10.1128/AAC.01321-06
Lisa MO, Kathryn RS, Jonathan DR, Heidi AS, Karl D, Robert JK, Hiroshi H (2010) Comparison of in vitro activities of fluoroquinolone-like 2,4- and 1,3-diones. Antimicrob Agents Chemother 54:3011–3014. doi:10.1128/AAC.00190-10
Buter TW, Fliri AFJ, Gallaschun RJ, Butler TW, Fliri AFJ, Gallaschun RJ (2002) Azabicycloalkane derivatives for use as serotonin reuptake inhibitors and \(5\text{-HT}_{2a}\) antagonists. EP 1178048, 2002-02-06
Seong C, Park N, Choi J, Park CM, Park W, Kong J (2008) Nover substituted-1H-quinazoline-2,4-dione derivatives, preparation method thereof and pharmaceutical composition containing the same. WO 2008004716, 2008-01-10
Allgeier H, Froestl W, Koller M, Mattes H, Nozulak J, Ofner S, Orain D, Rasetti V, Renaud J, Soldermann N, Floersheim P (2006) Quinazoline derivatives. WO 2006010591, 2006-02-02
Vittoria C, Daniela C, Flavia V, Ombretta L, Guido F, Chiara C, Alessandro G, Carla G, Nicoletta G, Paola G, Jacopo S, Francesca D, Stefano M (2006) Structural investigation of the 7-Chloro-3-hydroxy-1\(H\)-quinazoline-2,4-dione scaffold to obtain AMPA and Kainate receptor selective antagonists. synthesis, pharmacological, and molecular modeling studies. J Med Chem 49:6015–6026. doi: 10.1021/jm0604880
Daniela C, Ombretta L, Vittoria C, Flavia V, Daniela P, Guido F, Kurt L, Silvio O (2010) Pharmacological characterization of some selected 4,5-dihydro-4-oxo-1,2,4-triazolo[1,5-\(a\)]quinoxaline-2- carboxylates and 3-hydroxyquinazoline-2,4-diones as (\(S\))-2-amino-3-(3- hydroxy-5- methylisoxazol-4-yl)-propionic acid receptor antagonists. Chem Pharm Bull 58:908–911. doi:10.1248/cpb.58.908
Ronald KR, Jeffery BP, Richard AR, James JM, Robert F, Joan AK, David AB, Alfonso T (1988) Thiophene systems. 9. thienopyrimidinedione derivatives as potential antihypertensive agents. J Med Chem 31:1786–1793. doi:10.1021/jm00117a019
Shunsuke G, Hiroyuki T, Masami K, Koji M, Kooji K (2003) The process development of a novel aldose reductase inhibitor, FK366. Part 1. Improvement of discovery process and new syntheses of 1-substituted quinazolinediones. Org Process Res Dev 7:700–706. doi:10.1021/op0340661
Wu JJQ, Guo JX, Nguyen LT (2008) Novel agents of calcium ion channel modulators. WO 2008112715, 2008-09-18
Scarborough RM, Bauer SM, Pandey A (2008) Platelet ADP receptor inhibitors. WO 2008036843, 2008-03-27
Astles PC, Baker SR, Bonnefous C, Vernier JM, Keenan M, Sanderson AJ (2003) Aza-cyclic compounds as modulators of acetylcholine receptors. WO 03062224, 2003-07-31
Gou X, Lo HY, Man CC, Takanashi H (2009) Quinazolinedione cheymase inhibitors. WO 2009023655, 2009-02-19
Jérémie M, Bernard M, Caroline R, Corinne G, Jacques P, Raphaël F (2010) NF-\(\kappa \)B inducing kinase (NIK) inhibitors: identification of new scaffolds using virtual screening. Bioorg Med Chem Lett 20:4515–4520. doi: 10.1016/j.bmcl.2010.06.027
Gaudilliere B, Jacobelli H, Wilson MW, Picard JA (2003) Alkynylated fused ring pyrimidine compounds as matrix metalloprotease 13 inhibitors. WO 2003033478, 2003-04-24
Andrianjara C, Chantel-Barvian N, Gaudilliere B, Jacobelli H, Ortwine DF, Patt WC, Pham L, Kostlan CR, Wilson MW (2002) Quinazolines as MMP-13 inhibitors. WO 2002064572, 2002-08-22
Gaudilliere B, Jacobelli H, Wilson MW, Picard JA, Gaudilliere B, Jacobelli H, Wilson MW, Picard JA (2004) Preparation of new alkynylated quinazoline compounds as MMP-13 inhibitors. WO 2004007469, 2004-01-22
Dora C, Michelle AB, Cynthia JB, Saïd MS, Andrew DH (2005) Design, synthesis, and evaluation of potent and selective benzoyleneurea-based inhibitors of protein geranylgeranyl- transferase-I. Bioorg Med Chem 13:677–688. doi:10.1016/j.bmc.2004.10.053
Berdini V, Boyle RG, Saxty G, Verdonk ML, Woodhead SJ, Wyatt PG, Sore HF, Walker DW, Caldwell J, Collins I (2006) Phamaceutical compounds. WO 2006051290, 2006-05-18
Cortez R, Rivero I, Somanathan R, Aguirre G, Ramirez F, Hong E (1991) Synthesis of quinazolinedione using triphosgene. Synth Commun 21:285–292. doi:10.1080/00397919108020823
Sung JL, Yoshitaka K, Dingwei TY, Tamara AM, Christopher MR, Orest TM, Manton RF, Kigen K, Masafumi S (1995) Discovery of potent cyclic GMP phosphodiesterase inhibitors. 2-pyridyl- and 2-imidazolylquinazolines possessing cyclic GMP phosphodiesterase and thromboxane synthesis inhibitory activities. J Med Chem 38:3547–3557. doi:10.1021/jm00018a014
William FM, Joseph DS, Joseph WG, Adi MT, Carolyn AW, Mary FS, Elizabeth M, Chandra RS, Elizabeth B (1995) Novel inhibitors of the nuclear factor of activated T cells (NFAT)-mediated transcription of \(\beta \)-galactosidase: potential immunosupressive and antiinflammatory agents. J Med Chem 38:2557–2569. doi: 10.1021/jm00014a009
Edward BS (1985) Synthesis of quinazoline-2,4,5,8-(1H,3H)- tetrones and their amine nucleophilic addition chemistry. J Org Chem 50:4861–4865. doi:10.1021/jo00224a042
Takumi M, Noriaki O, Takatoshi I, Toshiyuki M (2000) Synthesis of 2,4-dihydroxyquinazolines using carbon dioxide in the presence of DBU under mild conditions. Tetrahedron Lett 41:1051–1053. doi:10.1016/S0040-4039(99)02231-5
Takumi M, Yoshio I (2002) Highly efficient synthesis of \(1H\)-quinazoline-2,4-diones using carbon dioxide in the presence of catalytic amount of DBU. Tetrahedron 58:3155–3158. doi:10.1016/S0040-4020(02)00279-X
Terrence JC, Patrick M, Sunil S (2005) An eco-efficient pilot plant scale synthesis of two 5-substituted-6,7-dimethoxy-1-H-quinazoline- 2,4-diones. Green Chem 7:586–589. doi:10.1039/B504305K
Gao J, He LN, Miao CX, Sébastien C (2010) Chemical fixation of \(\text{ CO}_{2}\): efficient synthesis of quinazoline-2,4(1H, 3H)-diones catalyzed by guanidines under solvent-free conditions. Tetrahedron 66:4063–4067. doi: 10.1016/j.tet.2010.04.011
Donald WC, Marianne SR (1989) 2,6-Dihydroxy -\(4H\)-pyridazino[3,4,5-de] quinazoline: a new ring system. J Heterocyclic Chem 26:1885–1886. doi: 10.1002/jhet.5570260667
Li Z, Huang H, Sun H, Jiang H, Liu H (2008) Microwave-assisted efficient and convenient synthesis of 2,4(1H,3H)-quinazolinediones and 2-thioxoquinazolines. J Comb Chem 10:484–486. doi:10.1021/cc800040z
Jack D, Abram NB, Jane M, Thomas NR (1986) Design and synthesis of 2-(arylamino)-4(3H)-quinazolinones as novel inhibitors of rat lens aldose reductase. J Med Chem 29:627–629. doi:10.1021/jm00155a007
Michael CW, Robert HS, Anthony JF, Robert LW (2006) Tandem palladium-catalyzed urea arylation-intramolecular ester amidation:regioselective synthesis of 3-alkylated 2,4-quinazolinediones. Org Lett 8:5089–5091. doi:10.1021/ol062009x
Wu H, Xie X, Liu G (2010) Parallel solution phase synthesis of \(3,6,7-4(3H)\)-quinazolinones and evaluation of their antitumor activities against human cancer. J Comb Chem 12:346–355. doi: 10.1021/cc900173s
Cai X, Zhai H, Wang J, Jeffrey F, Qu H, Yin L, Lai C, Bao R, Qian C (2010) Discovery of 7-(4-(3-Ethynylpenylamino)-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptanamide(CUDC-101) as a potent multi-acting HDAC, EGFR, and HER2 inhibitor for the treatment of cancer. J Med Chem 53:2000–2009. doi:10.1021/jm901453q
Stacy WR, Lidia CS, Peter A, Kenneth WB, Wendy DC, Michael AG, Kobporn LH, Manfred J, Paul K, Nancy T, Heather W (2002) Inhibitors of human histone deacetylase: synthesis and enzyme and cellular activity of straight chain hydroxamates. J Med Chem 45:753–757. doi:10.1021/jm015568c
Zhu N, Zhang F, Liu G (2010) Dynamic covalent chemistry of disulfides offers a highly efficient synthesis of diverse benzofused nitrogen-sulfur heterocycles in one pot. J Comb Chem 12:531–540. doi:10.1021/cc100042v
Li L, Liu G, Wang Z, Yuan Y, Zhang C, Tian H, Wu X, Zhang J (2004) Multi-step parallel synthesis of substituted 5-aminobenzimidazoles in solution-phase. J Comb Chem 6:811–821. doi:10.1021/cc049932f
Yan Y, Liu Z, Zhang J, Xu R, Hu X, Liu G (2011) A reverse method for diversity introduction of benzimidazole to synthesize H/K+-ATP enzyme inhibitors. Bioorg Med Chem Lett 21:4189–4192. doi:10.1016/j.bmcl.2011.05.080
Robert HS (2006) The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer 6:813–823. doi:10.1038/nrc1951
Aldo A, Silvia B, Massimiliano G, Alberto L, Alessandra L, Rita M, Mirella R, Lucilla V, Natalia C, Concettina C, Manuela V, Maddalena Z, Claudio S, Lanfranco M, Robert HS (2008) Antitumor activity of new substituted 3-(5-imidazo[2,1-b]- thiazolylmethylene)-2-indolinones and 3-(5-imidazo[2,1-\(b\)]thi- adiazolylmethylene)-2-indolinones: selectivity against colon tumor cells and effect on cell cycle-related \(\text{ events}^{1}\). J Med Chem 51:7508–7513. doi:10.1021/jm800827q
Michael RB, Kenneth DP (1995) Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev Res 34:91–109. doi:10.1002/ddr.430340203
Spasov AA, Yozhitsa IN, Bugaeva LI, Anisimova VA (1999) Benzimidazole derivatives: spectrum of pharmacological activity and toxicological properties (a review). Pharm Chem J 33:232–243. doi:10.1007/BF02510042
Fringuelli R, Milanese L, Schiaffella F (2005) Role of 1,4-benzothiazine derivatives in medicinal chemistry. Mini Rev Med Chem 5:1061–1073. doi:10.2174/138955705774933365
Honda T, Tajima H, Fujisawa K, Murai M, Aono H, Ban M (2008) Preparation of 1,4-benzothiazin-3-ones and related compounds for inhibiting angiogenesis. WO 2008053863, 2008-05-08
Eman MHA, Thoraya AF (2010) Synthesis, reactions, and biological activity of 1,4-benzothiazine derivatives. Monatsh Chem 141:661–667. doi:10.1007/s00706-010-0312-6
Washio Y, Kano K, Harris PA, Sato H, Mori I, West RI, Shibahara M, Toyoda H, Wang L, Nolte RT, Veal JM, Cheung M (2007) Discovery of novel benzimidazoles as potent inhibitors of TIE-2 and VEGFR-2 tyrosine kinase receptors. J Med Chem 50: 4453–4470. doi:10.1021/jm0611051
Rolf S, Alexander N, Christian DK (2006) Metal-mediated inhibition of Escherichia coli methionine aminopeptidase: structure–activity relationships and development of a novel scoring function for metal-ligand interactions. J Med Chem 49:511–522. doi:10.1021/jm050476z
Kristina S, Marijeta K, Katja E, Ivan S, Magdalena G, Krešimir P, Grace KZ (2007) Synthesis, antiviral and antitumor activity of 2-substituted-5-amidino-benzimidazoles. Bioorg Med Chem 15:4419–4426. doi:0.1016/j.bmc.2007.04.032
Ramla MM, Omar MA, Tokuda H, El-Diwani HI (2007) Synthesis and inhibitory activity of new benzimidazole derivatives against Burkitt’s lymphoma promotion. Bioorg Med Chem 15:6489–6496. doi:10.1016/j.bmc.2007.04.010
Acknowledgments
The research is financially supported by the National Natural Science Foundation of China (No. 81161120402) and National 863 Program of China (No. 2012AA020303).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, X., Xie, X. & Liu, G. Quinazoline-2,4(\(1H,3H\))-diones inhibit the growth of multiple human tumor cell lines. Mol Divers 17, 197–219 (2013). https://doi.org/10.1007/s11030-012-9421-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-012-9421-y