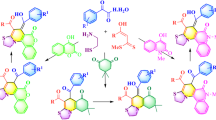
Abstract
A series of thiazoline-incorporated chalconoids, designed based on natural product scaffold, were efficiently synthesized via the reaction of 3-methyl-4-arylthiazole-2(3\(H\))-thiones and appropriate phenacyl halides, and subsequent desulfurization. The starting 3-methyl-4- arylthiazole-2(3\(H\))-thiones were also prepared from phenacyl halides. The structural aspects and (\(Z\))-geometry of compounds were confirmed by IR and \(^{1}\)H NMR spectral data. This chemistry provides a new library of compounds basically originated from phenacyl halides as building blocks, with potential activity for biomedical screening.







Similar content being viewed by others
References
Lim SS, Kim HS, Lee DU (2007) In vitro antimalarial activity of flavonoids and chalcones. Bull Korean Chem Soc 28:2495–2497
Dhar DN (1981) The chemistry of chalcones and related compounds. Wiley, New York
Lahtchev KL, Batovska DI, Parushev P, Ubiyvovk VM, Sibirny AA (2008) Antifungal activity of chalcones: a mechanistic study using various yeast strains. Eur J Med Chem 43:2220–2228. doi:10.1016/j.ejmech.2007.12.027
López SN, Castelli M, Zacchino SA, Domínguez JN, Lobo G, Jaime CC, Cortés JCG, Ribas JC, Devia C, Rodríguez AM, Enriz RD (2001) In vitro antifungal evaluation and structure–activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg Med Chem 9:1999–2013. doi:10.1016/S0968-0896(01)00116-X
Ko HH, Tsao LT, Yu KL, Liu CT, Wang JP, Lin CN (2003) Structure–activity relationship studies on chalcone derivatives: the potent inhibition of chemical mediators release. Bioorg Med Chem 11:105–111. doi:10.1016/S0968-0896(02)00312-7
Matsuda H, Morikawa T, Ando S, Iwao T, Masayuki Y (2003) Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg Med Chem 11:1995–2000. doi:10.1016/S0968-0896(03)00067-1
Narender T, Shweta, Tanvir K, Rao MS, Srivastava K, Puri SK (2005) Prenylated chalcones isolated from Crotalaria genus inhibits in vitro growth of the human malaria parasite Plasmodium falciparum. Bioorg Med Chem Lett 15:2453–2455. doi:10.1016/j.bmcl.2005.03.081
Nazarian Z, Emami S, Heydari S, Ardestani SK, Nakhjiri M, Poorrajab F, Shafiee A, Foroumadi A (2010) Novel antileishmanial chalconoids: synthesis and biological activity of 1- or 3-(6-chloro-2H-chromen-3-yl)propen-1-ones. Eur J Med Chem 45:1424–1429. doi:10.1016/j.ejmech.2009.12.046
Foroumadi A, Emami S, Sorkhi M, Nakhjiri M, Nazarian Z, Heydari S, Ardestani SK, Poorrajab F, Shafiee A (2010) Chromene-based synthetic chalcones as potent antileishmanial agents: synthesis and biological activity. Chem Biol Drug Des 75:590–596. doi:10.1111/j.1747-0285.2010.00959.x
Wu J, Wang X, Yi Y, Lee K (2003) Anti-AIDS agents 54. A potent anti-HIV chalcone and flavonoids from genus Desmos. Bioorg Med Chem Lett 13:1813–1815. doi:10.1016/S0960-894X(03)00197-5
Nakhjiri M, Safavi M, Alipour E, Emami S, Atash AF, Jafari-Zavareh M, Ardestani SK, Khoshneviszadeh M, Foroumadi A, Shafiee A (2012) Asymmetrical 2, 6-bis(benzylidene)cyclohexanones: synthesis, cytotoxic activity and QSAR study. Eur J Med Chem 50:113–123. doi:10.1016/j.ejmech.2012.01.045
Firoozpour L, Edraki N, Nakhjiri M, Emami S, Safavi M, Ardestani SK, Khoshneviszadeh M, Shafiee A, Foroumadi A (2012) Cytotoxic activity evaluation and QSAR study of chromene-based chalcones. Arch Pharm Res 35: 2117–2125. doi:10.1007/s12272-012-1208-2
Ducki S, Forrest R, Hadfield JA, Kendall A, Lawrence NJ, Mcgown AT, Rennison D (1998) Potent antimitotic and cell growth inhibitory properties of substituted chalcones. Bioorg Med Chem Lett 8:1051–1056. doi:10.1016/S0960-894X(98)00162-0
Parmar VS, Sharma NK, Husain M, Watterson AC, Kumar J, Samuelson LA, Ashok LC, Prasad AK, Kumar A, Malhotra S, Kumar N, Jha A, Singh A, Singh I, Himanshu VA, Shakil NA, Trikha S, Mukherjee S, Sharma SK, Singh SK, Kumar A, Jha HN, Olsen CE, Stove CP, Bracke ME, Mareel MM (2003) Synthesis, characterization and in vitro anti-invasive activity screening of polyphenolic and heterocyclic compounds. Bioorg Med Chem 11:913–929. doi:10.1016/S0968-0896(02)00539-4
Won S, Liu C, Tsao L, Weng J, Ko H, Wang J, Lin C (2005) Synthetic chalcones as potential anti-inflammatory and cancer chemopreventive agents. Eur J Med Chem 40:103–112. doi:10.1016/j.ejmech.2004.09.006
Shi HB, Zhang SJ, Ge QF, Guo DW, Cai CM, Hu WX (2010) Synthesis and anticancer evaluation of thiazolyl-chalcones. Bioorg Med Chem Lett 20:6555–6559. doi:10.1016/j.bmcl.2010.09.041
Insuasty B, Tigreros A, Orozco F, Quiroga J, Abonía R, Nogueras M, Sanchez A, Cobo J (2010) Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg Med Chem 18:4965–4974. doi:10.1016/j.bmc.2010.06.013
Bandgar BP, Gawande SS, Bodade RG, Gawande NM, Khobragade CN (2009) Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg Med Chem 17:8168–8173. doi:10.1016/j.bmc.2009.10.035
Meyers C, Yáñez M, Elmaatougi A, Verhelst T et al (2008) 2-Substituted 4-, 5-, and 6-[(1E)-3-oxo-3-phenylprop-1-en-1-yl]pyridazin-3(2H)-ones and 2-substituted 4,5-bis[(1\(E)\)-3-oxo-3-phenylprop-1-en-1-yl]pyridazin-3(2H)-ones as potent platelet aggregation inhibitors: design, synthesis, and SAR studies. Bioorg Med Chem Lett 18:793–797. doi:10.1016/j.bmcl.2007.11.034
Romagnoli R, Baraldi PG, Carrion MD, Cara CL, Cruz-Lopez O, Preti D (2008) Design, synthesis and biological evaluation of thiophene analogues of chalcones. Bioorg Med Chem 16:5367–5376. doi:10.1016/j.bmc.2008.04.026
Kumar D, Kumar NM, Akamatsu K, Kusaka E, Harada H, Ito T (2010) Synthesis and biological evaluation of indolyl chalcones as antitumor agents. Bioorg Med Chem Lett 20:3916–3919. doi:10.1016/j.bmcl.2010.05.016
Ortega-Alfaro MC, López-Cortés JG, Sánchez HR, Toscano RA, Carrillo GP, Álvarez-Toledano C (2005) Improved approaches in the synthesis of new 2-(1,3-thiazolidin-2Z-ylidene)acetophenones. ARKIVOC vi:356–365.
Rudorf WD, Schierhorn A, Augustin M (1979) Reaktionen von o-halogenacetophenonen mit schwefelkohlenstoff und phenylisothiocyanat. Tetrahedron 35:551–556. doi:10.1016/0040-4020(79)80155-6
Emami S, Hosseinimehr SJ, Shahrbandi K, Enayati AA, Esmaeeli Z (2012) Synthesis and evaluation of 2(3H)-thiazole thiones as tyrosinase inhibitors. Arch Pharm 345:629–637. doi:10.1002/ardp.201200028
Acknowledgments
This work was supported by a grant from the Research Council of Mazandaran University of Medical Sciences, Sari, Iran
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ayati, A., Emami, S. Straightforward synthesis of thiazoline-incorporated chalconoids from phenacyl halides. Mol Divers 17, 41–47 (2013). https://doi.org/10.1007/s11030-012-9416-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-012-9416-8