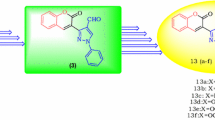
Abstract
An efficient three-step approach was developed to assemble indole- or benzofuran-fused benzocarbazole-1,4-diones in 42–53% overall yield. This approach includes AgOAc-promoted oxidative cyclization of 2,6-di-bromocyclohexene-1,4-dione with indol-3-ylpropanoid acid, condensation of the resulting bromocarbazole intermediates with phenols or anilines, followed by Pd(OAc)-catalyzed cyclization. Such convenient synthetic protocol and the novelty of the corresponding products will largely assist our drug design and development program.
Graphical Abstract

Similar content being viewed by others
References
Knölker HJ, Reddy KR (2002) Isolation and synthesis of biologically active carbazole alkaloids. Chem Rev 102: 4303–4428. doi:10.1021/cr020059j
Knölker HJ, Reddy KR (2008) Chemistry and biology of carbazole alkaloids. Alkaloids Chem Biol 65: 1–410
Asche C, Demeunynck M (2007) Antitumor carbazoles. Anti Cancer Agents Med Chem 2: 247–267
Thevissen K, Marchand A, Chaltin P, Meert EMK, Cammue BPA (2009) Antifungal carbazoles. Curr Med Chem 16: 2205–2211
Nettleton DE, Doyle TW, Krishnan B, Matsumoto GK, Clardy J (1985) Isolation and structure of rebeccamycin—a new antitumor antibiotic from nocardia aerocoligenes. Tetrahedron Lett 26: 4011–4014
Bush JA, Long BH, Catino JJ, Bradner WT, Tomita K (1987) Production and biological activity of rebeccamycin, a novel antitumor agent. J Antibiot 40: 668–678
Bailly C, Riou JF, Colson P, Houssier C, Rodrigues-Pereira E, Prudhomme M (1997) DNA cleavage by topoisomerase I in the presence of indolocarbazole derivatives of rebeccamycin. Biochemistry 36: 3917–3929. doi:10.1021/bi9624898
Moreau P, Anizon F, Sancelme M, Prudhomme M, Severe D, Riou JF, Goosens JF, Henichart JP, Bailly C, Labourier E, Tazzi J, Fabbro D, Meyer T, Aubertin AM (1999) Synthesis, mode of action, and biological activities of rebeccamycin bromo derivatives. J Med Chem 42: 1816–1822. doi:10.1021/jm980702n
Anizon F, Belin L, Moreau P, Sancelme M, Voldoire A, Prudhomme M, Ollier M, Severe D, Riou JF, Bailly C, Fabbro D, Meyer T (1997) Syntheses and biological activities (Topoisomerase inhibition and antitumor and antimicrobial properties) of rebeccamycin analogues bearing modified sugar moieties and substituted on the imide nitrogen with a methyl group. J Med Chem 40: 3456–3465. doi:10.1021/jm9702084
Moreau P, Anizon F, Sancelme M, Prudhomme M, Bailly C, Carrasco C, Ollier M, Severe D, Riou JF, Fabbro D, Meyer T, Aubertin AM (1998) Syntheses and biological evaluation of indolocarbazoles, analogues of rebeccamycin, modified at the imide heterocycle. J Med Chem 41: 1631–1640. doi:10.1021/jm970843+
Gilbert EJ, Chisholm JD, Van Vranken DL. (1999) Conformational control in the rebeccamycin class of indolocarbazole glycosides. J Org Chem 64: 5670–5676. doi:10.1021/jo990296v
Omura S, Iwai Y, Hirano A, Nakagawa A, Awaya J, Tsuchya H, Takahashi Y, Masuma R (1977) A new alkaloid AM-282 of streptomyces origin taxonomy, fermentation, isolation and preliminary characterization. J Antibiot 30: 275–282
Ruegg UT, Burgess GM (1989) Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci 10: 218–220
Prudhomme M (2004) Biological targets of antitumor indolocarbazoles bearing a sugar moiety. Curr Med Chem Anticancer Agents 4: 509–521
Akinaga S, Sugiyama K, Akiyama T (2000) UCN-01 (7-hydroxystaurosporine) and other indolocarbazole compounds: a new generation of anti-cancer agents for the new century?. Anticancer Drug Des 15: 43–52
Hotte SJ, Oza A, Winquist EW, Moore M, Chen EX, Brown S, Pond GR, Dancey JE, Hirte HW (2006) Phase I trial of UCN-01 in combination with topotecan in patients with advanced solid cancers: a Princess Margaret Hospital Phase II Consortium study. Ann Oncol 17: 334–340. doi:10.1093/annonc/mdj076
Jiang X, Zhao B, Britton R, Lim LY, Leong D, Sanghera JS, Zhou BBS, Piers E, Andersen RJ, Roberge M (2004) Inhibition of Chk1 by the G 2 DNA damage checkpoint inhibitor isogranulatimide. Mol Cancer Ther 3: 1221–1227
Conchon E, Anizon F, Aboab B, Prudhomme M (2007) Synthesis and biological activities of new checkpoint kinase 1 inhibitors structurally related to granulatimide. J Med Chem 50: 4669–4680. doi:10.1021/jm070664k
Conchon E, Anizon F, Aboab B, Golsteyn RM, Leonce S, Pfeiffer B, Prudhomme M (2008) Synthesis, checkpoint kinase 1 inhibitory properties and in vitro antiproliferative activities of new pyrrolocarbazoles. Bioorg Med Chem 16: 4419–4430. doi:10.1016/j.bmc.2008.02.061
Henon H, Messaoudi S, Anizon F, Aboab B, Kucharczyk N, Leonce S, Golsteyn RM, Pfeiffer B, Prudhomme M (2007) Bis-imide granulatimide analogues as potent checkpoint 1 kinase inhibitors. Eur J Pharmcol 554: 106–112. doi:10.1016/j.ejphar.2006.10.022
Henon H, Anizon F, Golsteyn RM, Leonce S, Hofmann R, Pfeiffer B, Prudhomme M (2006) Synthesis and biological evaluation of new dipyrrolo[3,4-a:3,4-c]carbazole-1,3,4,6-tetraones, substituted with various saturated and unsaturated side chains via palladium catalyzed cross-coupling reactions. Bioorg Med Chem 14: 3825–3834. doi:10.1016/j.bmc.2006.01.030
Sanchez-Martinez C, Shih C, Faul MM, Zhu G, Paal M, Somoza C, Li T, Kumrich CA, Winneroski LL, Xun Z (2003) Aryl[a]pyrrolo[3,4-c]carbazoles as selective cyclin D1-CDK4 inhibitors. Bioorg Med Chem Lett 13: 3835–3839. doi:10.1016/j.bmcl.2004.04.088
Richards RW, Rothschild JM, Willis AC, de Chazal NM, Kirk K, Saliba KJ, Smith GD (1999) Calothrixins A and B, novel pentacyclic metabolites from calothrix cyanobacteria with potent activity against malaria parasites and human cancer cells. Tetrahedron 55: 13513–13520
Sissouma D, Maingot L, Collet S, Guingant A (2006) Concise and efficient synthesis of calothrixin B. J Org Chem 71: 8384–8389. doi:10.1021/jo061270o
Sanchez C, Mendez C, Salas JA (2006) Indolocarbazole natural products: occurrence, biosynthesis, and biological activity. Nat Prod Rep 23: 1007–1045. doi:10.1039/b601930g
Henon H, Conchon E, Hugon B, Messaoudi S, Golsteyn RM, Prudhomme M (2008) Pyrrolocarbazoles as checkpoint 1 kinase inhibitors. Anti Cancer Agents Med Chem 8: 577–597
Conchon E, Anizon F, Golsteyn RM, Leonce S, Pfeiffer B, Prudhomme M (2006) Synthesis, in vitro antiproliferative activities, and Chk1 inhibitory properties of dipyrrolo[3,4-a:3,4-c]carbazole-triones. Tetrahedron 62: 11136–11144. doi:10.1016/j.tet.2006.09.027
Henon H, Anizon F, Kucharczyk N, Loynel A, Casara P, Pfeiffer B, Prudhomme M (2006) Expedited synthesis of substituted dipyrrolo[3,4-a:3,4-c]carbazole-1,3,4,6-tetraones structurally related to granulatimide. Synthesis 4: 711–715
Henon H, Anizon F, Pfeiffer B, Prudhomme M (2006) Synthesis of dipyrrolo[3,4-a:3,4-c]carbazole-1,3,4,6-tetraones bearing a sugar moiety. Tetrahedron 62: 1116–1123. doi:10.1016/j.tet.2005.10.077
Henon H, Messaoudi S, Hugon B, Anizon F, Pfeiffer B, Prudhomme M (2005) Synthesis of granulatimide bis-imide analogues. Tetrahedron 61: 5599–5614. doi:10.1016/j.tet.2005.03.101
Caballero E, Alonso D, Pelaez R, Alvarez C, Puebla P, Sanz F, Medarde M, Tome F (2004) 1-Phthalimido-4-(3-indolyl)-2-siloxy-1,3-butadienes: synthesis and Diels–Alder reactivity. Tetrahedron Lett 45: 1631–1634. doi:10.1016/j.tetlet.2003.12.118
Yoshida T, Nishiyachi M, Nakashima N, Murase M, Kotani E (2003) Synthesis of granulatimide positional analogues. Chem Pharm Bull 51: 209–214. doi:10.1248/cpb.51.209
Hugon B, Pfeiffer B, Renard P, Prudhomme M (2003) Synthesis of granulatimide analogues bearing a maleimide instead of an imidazole heterocycle. Tetrahedron Lett 44: 3935–3937. doi:10.1016/S0040-4039(03)00823-2
Yoshida T, Nishiyachi M, Nakashima N, Murase M, Kotani E (2002) New synthetic route to granulatimide and its structural analogues. Chem Pharm Bull 50: 872–876. doi:10.1248/cpb.50.872
Ding CY, Tu SH, Li FY, Wang YX, Yao QZ, Hu WX, Xie H, Meng LH, Zhang A (2009) Synthesis study on marmycin a: preparation of the C3′-desmethyl analogues. J Org Chem 74: 6111–6119. doi:10.1021/jo9011078
Anderson JM, Kochi JK (1970) Silver(I)-catalyzed oxidative decarboxylation of acids by peroxydisulfate. Role of silver (II). J Am Chem Soc 92: 1651–1659. doi:10.1021/ja00709a039
Minisci F (1973) Novel applications of free-radical reactions in preparative organic chemistry. Synthesis 5: 1–24
Cowden CJ (2003) Use of N-protected amino acids in the minisci radical alkylation. Org Lett 5: 4497–4499. doi:10.1021/ol801736h
Ding CY, Tu SH, Yao QZ, Li FL, Wang YX, Hu WX, Zhang A (2009) One-pot three-step synthesis of naphtho[2,3-a]carbazole-5,13-diones using tandem radical alkylation-cyclization-aromatization reaction sequence. Adv Synth Catal (adsc.200900789, doi:10.1002/adsc.200)
Rogge M, Fischer G, Pindur U, Schollmeyer D (1996) [α]-anellated carbazoles with antitumor activity: synthesis and cytotoxicity. Monatsh Chem 127: 97–102
Pindur U, Marotto A, Schulze E, Fischer G (2000) Functionalized and [a]-anellated carbazoles as potential B-DNA ligands: experimental studies of DNA binding and molecular modelling of intercalation complexes. Pharmazie 55: 717–732
Perumal PT, Bhatt MV (1979) A new method for the conversion of halophenols and halonaphthols to quinnones. Synthesis 3: 205–206
Bedford RB, Betham M, Charmant JPH, Weeks AL (2008) Intramolecular direct arylation in the synthesis of fluorinated carbazoles. Tetrahedron 64: 6038–6050. doi:10.1016/j.tet.2008.01.143
Bolognesi ML, Lizzi F, Perozzo R, Brunc R, Cavallia A (2008) Synthesis of a small library of 2-phenoxy-1,4-naphthoquinone and 2-phenoxy-1,4-anthraquinone derivatives bearing anti-trypanosomal and anti-leishmanial activity. Bioorg Med Chem Lett 18: 2272–2276. doi:10.1016/j.bmcl.2008.03.009
Narayan S, Roush WR (2004) Studies toward the total synthesis of angelmicin B (hibarimicin B): synthesis of a model CD-D′ arylnaphthoquinone. Org Lett 6: 3789–3792. doi:10.1021/ol0613822
Hagelin H, D Oslob J, Akermark B (1999) Oxygen as oxidant in palladium-catalyzed inter- and intramolecular coupling reactions. Chem Eur J 5: 2413–2416. doi:10.1002/(SICI)1521-3765(19990802)5:8<2413
Stuart DR, Villemure E, Fagnou K (2007) Elements of regiocontrol in palladium-catalyzed oxidative arene cross-coupling. J Am Chem Soc 129: 12072–12073. doi:10.1021/ja0745862
Tietze LF, Singidi RR, Gericke KM (2007) Total synthesis of the proposed structure of the anthrapyran metabolite δ-indomycinone. Chem Eur J 13: 9939–9947. doi:10.1002/chem.200700823
Author information
Authors and Affiliations
Corresponding author
Additional information
Part of this study has been presented in the 2nd International Symposium on Combinatorial Sciences in Biology, Chemistry, Catalysts and Materials in Beijing on September 19–23, 2009.
Rights and permissions
About this article
Cite this article
Tu, S., Ding, C., Hu, W. et al. Facile synthesis of indole- or benzofuran-fused benzo[a]carbazole-1,4-diones using a tandem two-step reaction sequence. Mol Divers 15, 91–99 (2011). https://doi.org/10.1007/s11030-010-9241-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-010-9241-x