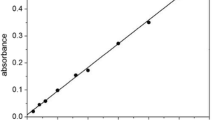
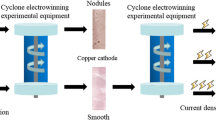
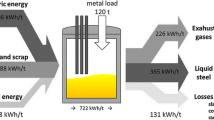
No less than 90% of copper-containing ore extracted and processed by pyrometallurgical methods is currently subjected to electrolytic refining to obtain copper cathode. Improvement in the electrolytic refining process of copper to obtain products for the electrotechnical industry can be related to the extraction of precious metals from copper electrolytic sludge formed during the process. This solves the problem of obtaining a 99.99% copper cathode with the lowest impurity content, highest electrical conductivity, and excellent mechanical properties. During the electrolytic refining process, dendritic cohesion occurs between the electrodes, which lead to short circuits and power losses. The dendritic trebles between the anode and the cathode are destroyed mechanically and converted into sludge, accumulating at the bottom of the electrolytic cell as sludge with high concentrations of silver, selenium, tellurium, and other valuable elements. In the course of research on the electrolytic unit, the causes of cohesion between the electrodes were established, and the formation process of the three types of dendritic precipitation was analyzed. Measures associated with the control and optimization of the control system of electrolytic refining need to be developed to reduce the negative influence of short circuits with the solution to questions of the maximum transfer of precious and rare metals in slime. Additional control parameters are proposed to be introduced in case of deviations from technology related to the monitoring and registration of short circuits and the level of copper electrolyte precipitate in the cell to optimize the automation system in controlling the temperature regime and the electrolyte composition through the digital twin software. The measures developed reduce the specific power consumption and increase the efficiency of copper cathode production while extracting functional components from copper electrolyte sludge.









Similar content being viewed by others
References
V. Litvinenko, I. Bowbrick, I. Naumov, and Z. Zaitseva, “Global guidelines and requirements for professional competencies of natural resource extraction engineers: Implications for ESG principles and sustainable development goals,” J. Clean. Prod., 338 (130530), 2–9 (2022); https://doi.org/10.1016/j.jclepro.2022.130530.
V. S. Litvinenko, “Correction to: Digital economy as a factor in the technological development of the mineral sector,” Nat. Resour. Res., 29, No. 3, 1521–1541 (2020); 10.1007%2Fs11053-019-09568-4.
A. V. Veikum, and V. S. Zhaglov, “Purification of copper electrolyte from arsenic,” Molod. Uchen., No. 15 (305), 92–94 (2020); [Electronic resource]: https://moluch.ru/archive/305/68735/ (accessed 08.10.2022).
V. Yu. Bazhin and H. H. Nguyen, “Vietnamese metallurgy on the way out of the crisis with the use of automated control systems,” AIP Conf. Proc., 2467, No. 1, AIP Publishing LLC (2022); https://doi.org/10.1063/5.0092750.
O. Devos, C. Gabrielli, L. Beitone, C. Mace, E. Ostermann, and H. Perrot, “Growth of electrolytic copper dendrites. I: Current transients and optical observation,” J. Electroanal. Chem., 606, No. 2, 75–84 (2007); https://doi.org/10.1016/j.jelechem.2007.03.028.
O. Devos, C. Gabrielli, L. Beitone, C. Mace, E. Ostermann, and H. Perrot, “Growth of electrolytic copper dendrites. II: Oxalic acid medium,” J. Electroanal. Chem., 606, No. 2, 85–94 (2007); https://doi.org/10.1016/j.jelechem.2007.05.003.
O. Devos, C. Gabrielli, L. Beitone, C. Mace, E. Ostermann, and H. Perrot, “Growth of electrolytic copper dendrites. III: Influence of the presence of copper sulphate,” J. Electroanal. Chem., 606, No. 2, 95–102 (2007); 10.1016/j. jelechem.2007.05.002.
M. Z. Mubarok, I. Filzwieser, and P. Paschen, “Dendritic cathode growth during copper electrorefining in the presence of solid particles,” Erzmetall, 58, No. 6, 315–329 (2005).
M. C. Ma, G. Li, X. Chen, L. A. Archer, and J. Wan, “Suppression of dendrite growth by cross-flow in microfluidics,” Sci. Adv., 7, No. 8, 1–8 (2021); https://doi.org/10.1126/sciadv. abf6941.
J.-H. Han, E. Khoo, P. Bai, and M. Z. Bazant, “Overlimiting current and control of dendritic growth by surface conduction in nanopores,” Sci. Rep., 4, No. 1, 1–8 (2014); https://doi.org/10.1038/srep07056.
R. K. Astakhova, A. B. Belenkiy, B. S. Krasikov, Yu. E. Kudryashov, A. E. Lebedev, E. M. Solovyov, and S. V. Yakovleva, “Behavior of some surfactants under conditions of copper electrorefining (dependence of polarization effects caused by addition of surfactants on their concentration in solution),” ZhPH, No. 5, 1028–1032 (1990).
A. K. Shestakov, P. A. Petrov, and M. Yu. Nikolaev, “Automatic system for detecting visible outliers in electrolysis shop of aluminum plant based on technical vision and a neural network,” Metallurg, No. 10, 105–112 (2022); DOI https://doi.org/10.52351/00260827_2022_10_105.
Quang Phuc Le, P. N. Dmitriev, Van Zui Than, and Yunpeng Li, “Influence of the main roof on the parameters of the bearing pressure zone in the marginal part of the formation,” Gor. Informats.-Anal. Byull., 6, No. 1, 68–82 (2022); https://doi.org/10.25018/0236_1493_2022_61_0_68.
D. R. Gabe and D. J. Robinson, “Department of Metallurgy, University of Sheffield. Dendritic formation and the effects of addition agents in the electrodeposition of copper from acid sulphate solutions,” J. Surf Eng Coat., 49, 17–21 (1971).
W. Shao, G. Zangari, “Dendritic growth and morphology selection in copper electrodeposition from acidic sulfate solutions containing chlorides,” J. Phys. Chem. C., 113, 97–102 (2009).
P. E. Aqueveque, E. P. Wiechmann, J. Herrera, and E. J. Pino, “Measurable variables in copper electrowinning and their relevance to predicting process performance,” IEEE Trans. Ind. Appl., 51, No. 3, 2607–2614 (2015); https://doi.org/10.1109/tia.2014.2377298.
E. P. Wiechmann, P. Aqueveque, J. A. Henriquez, L. G. Munoz, and A. S. Morales, “BMC: A modulating bar for copper electrowinning designed for heavy duty and high reliability,” IEEE Trans. Ind. Appl., 50, No. 4, 2375–2381 (2014); https://doi.org/10.1109/tia.2013.2290841.
E. P. Wiechmann, A. S. Morales, and P. Aqueveque, “Full measuring system for copper electrowinning processes using optibar intercell bars,” IEEE Trans. Ind. Appl., 45, No. 5, 1575–1582 (2009); https://doi.org/10.1109/tia.2009.2027151.
Do-it-Yourself Thermal Imager. Camera-based Thermal Imager. The Principle of Operation of the Thermal Imager (2018); [Electronic resource] https://www.syl.ru/article/179314/new_teplovizor-svoimi-rukami-teplovizor-iz-fotoapparata-printsip-rabotyiteplovizora (accessed 10/11/2022).
S.V. Medveditsin, Pat. 479818 RF, M. Cl. C 22d 3/02, Device for Detecting Short Circuits in Electrolysis Baths during Electrolytic Refining, Applicant Ural Branch of Institute “Tsvetmetavtomatika”, No. 962877/22-1; Submitted 10/02/73; Publ. 08/05/75, Bull. No. 29.
Yu. R. Sevumyan, I. N. Mikhailov, M. I. Filimonov, and D. V. Pivovarov, Pat. 675084 RF, M. Cl. 2 C 22 V 15/14, A Method for Detecting Short Circuits in Copper Electrolysis Baths, Applicant All-Union Scientific Research and Construction Institute “Tsvetmetavtomatika”, No. 2582850/22-02; submitted 02/17/78; Publ. 07/25/79, Bull. No. 27.
Y. Zhao, B. Zhang, W. Chen, H. Wang, M. Wang, and H. Hou, “Simulation for the influence of interface thickness on the dendritic growth of nickel-copper alloy by a phase-field method,” ES Mater. Manuf., 2, No. 2, 45–50 (2018); https://doi.org/10.30919/esmm5f135.
J. H. Han, E. Khoo, and P. Bai, “Over-limiting current and control of dendritic growth by surface conduction in nanopores,” Sci Rep 4, 7056, 1–8 (2014); https://doi.org/10.1038/srep07056.
H. Miyaharaand K. Ogi, “Thermal and solutal influences of continuous fibers on matrix dendrite growth in composite materials,” Mater. Trans., 42, No. 2, 258–262 (2001); https://doi.org/10.2320/matertrans.42.258.
A. G. Syrkov, V. R. Kabirov, A. P. Pomogaibin, and N. K. Kkhan, “Electrophilic-nucleophilic properties as a factor in the formation of antifriction and hydrophobic properties of surface-modified metals with ammonium and organosilicon compounds,” Condens. Matter Interphases, 23, No. 2, 282–290 (2021); https://doi.org/10.17308/kcmf.2021.23/3478.
R. F. M. Van Doremalen, J. J. Van Netten, J. G. Van Baal, M. M. R. Vollenbroek-Hutten, and F. Van Der Heijden, “Validation of low-cost smartphone-based thermal camera for diabetic foot assessment,” Diabetes Res. Clin. Pract., 149, 132–139 (2019); https://doi.org/10.1016/j.diabres.2019.01.032.
K. Bun, R. Fraser, H. Garces Baron, et al., Pat. No. 2641289 C2 RF, IPC C25B 15/02, H02J 7/04, C25C 7/06, Improved Electric Current Measurement and Control System for Electrolysis Shops, No. 2015110618, Submitted 08/26/2013, Publ. 01/17/2018; applicant HETCH PTI LTD, EDN PFPNCM.
J. M. Sonawane, D. Pant, P. C. Ghosh, and S. B. Adeloju, “Polyaniline-copper composite: A non-precious metal cathode catalyst for low-temperature fuel cells,” Energy Fuels, 35, No. 4, 3385–3395 (2021); https://doi.org/10.1021/acs.energyfuels.0c04152.
Y. Bian, L. Su, Z. Yu, Z. Lv, H. Chen, Y. Zhou, C. Meng, H. Du, M. Cai, H. Cao, and M.-C. Lin, “Graphite/copper phthalocyanine composite cathode for overcharge protection and gas evolution suppression in aluminum-ion batteries at room temperature,” Electrochim. Acta, 135188, 1–23 (2019); https://doi.org/10.1016/j.electacta.2019.135188.
Patent No. 2233913 C1 RF, IPC C25B 15/02, H02J 7/04, C25C 7/06, Method for Electrolytic Refining of Copper, No. RU2003101026/02A, Submitted 01/14/2003, Publ. 08/10/2004, Mining and Metallurgical Company Norilsk Nickel.
Patent No. 2233913 C1 RF, IPC C25B 15/02, H02J 7/04, C25C 7/06, Method for Electrolytic Refining of Copper: No. RU2003101026/02A, Submitted 01/14/2003, Publ. 08/10/2004, Mining and Metallurgical Company Norilsk Nickel.
A. K. Shestakov, R. M. Sadykov, and P. A. Petrov, “Multifunctional crust breaker for automatic aluminum feeding system of aluminum reduction cell,” E3S Web Conf., 266, 1–12 (2021); https://doi.org/10.1051/e3sconf/202126609002.
I. I. Beloglazov, D. S. Sabinin, and M. Yu. Nikolaev, “Modeling the disintegration process for ball mills using dem,” MIAB. Mining Inf. Anal. Bull., 6, No. 2, 268–282 (2022); https://doi.org/10.25018/0236_1493_2022_62_0_268.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 67, No. 1, pp. 49–56, January, 2023. Russian DOIhttps://doi.org/10.52351/00260827_2023_01_49.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, H.H., Bazhin, V.Y. Optimization of the Control System for Electrolytic Copper Refining with Digital Twin During Dendritic Precipitation. Metallurgist 67, 41–50 (2023). https://doi.org/10.1007/s11015-023-01487-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-023-01487-3