Abstract
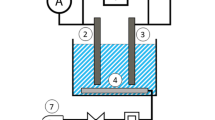
Copper wastewater from industry is detrimental to plants and environment. There are some problems in the aspects of high efficiency and energy saving during treatment of wastewater. In present work, the novel double iron electrodes technique of pulse-alternating current was applied to flocculate copper in wastewater. The process parameters of the copper removal in wastewater were studied in the developed electrochemical reactors. Scanning electron microscopy, X-ray diffraction, and energy-dispersive spectroscopy were used to characterize the electrocoagulations. The copper residue in the effluent was measured by UV spectrophotometry. The adsorption mechanism was described through the isothermal adsorption curves of copper during flocculation processes. The simulated wastewater containing 100 mg dm−3 Cu2+ and 100 mg dm−3 NaCl as conductive salt was adjusted to pH 7.8–8 with ammonia or sulfuric acid. At room temperature of 20–25 °C, controlling the flow rate of 3 dm3 min−1, and applying pulse-alternating current of 40 μA gFe −1, the copper residue in the effluent passing through four-series reactors was reduced to 0.118 mg dm−3, which was far lower than 0.3 mg dm−3 (from GB20900–2008). The removal rate of copper could reach 99.882%. The removal of copper in the wastewater treated via our electrocoagulation technique was far more efficient than the conventional DC current coagulation and chemical flocculation. The double iron electrodes were used to reduce the concentration polarization and improved the current efficiency. The significant economic and good social benefit will be promisingly produced if our developed technique is applied in the treatment of industrial wastewater.













Similar content being viewed by others
References
Dave RS, Dave GB, Mishra VP (2011) Removal of nickel from electroplating wastewater by weakly basic chelating anion exchange resins: DOWEX 50x4, DOWEX 50x2 and DOWEX M-4195. Journal of Applied Sciences in Environmental Sanitation 6(1):39–44
Fanni D, Fanos V, Gerosa C, Piras M, Dessi A, Atzei A, Van EP, Gibo Y, Faa G (2014) Effects of iron and copper overload on the human liver: an ultrastructural study. Curr Med Chem 21:3768–3774
Guo Y, Shao Q (2007) Integrated treatment and reuse of wastewater from printed circuit board production. Industrial Water Treatment 27:70–73
He X, Fang Z, Jia J, Ma L, Li Y, Chai Z, Chen X (2014) Study on the treatment of wastewater containing Cu(II) by D851 ion exchange resin. Desalin Water Treat 57:3597–3605
Igwe JC, Abia AA (2014) A bioseparation process for removing heavy metals from waste water using biosorbents. Afr J Biotechnol 5:1167–1179
Ishizaki H, Spitzer M, Wildenhain J, Anastasaki C, Zeng Z, Dolma S, Shaw M, Madsen E, Gitlin J, Marais R (2010) Combined zebrafish-yeast chemical-genetic screens reveal gene-copper-nutrition interactions that modulate melanocyte pigmentation. Dis Model Mech 3:639–651
Jeong BH, Hoek EMV, Yan Y, Subramani A, Huang X, Hurwitz G, Ghosh AK, Jawor A (2007) Interfacial polymerization of thin film nanocomposites: a new concept for reverse osmosis membranes. J Membr Sci 294:1–7
Jiang C, Xuemei LI, Aixian SU (2011) Study of copper extraction recycling process from alkaline etching of printed circuit board. Chem Ind Eng Progr (s2):122–125
Jin-sheng S, Lei S (2009) Studying and carrying out GB21900-2008《 The discharge standard for pollutants from electroplating industry》 (I) (to be continued). Plating & Finishing 7:40–42
Ju F, Hu Y (2011) Removal of EDTA-chelated copper from aqueous solution by interior microelectrolysis. Sep Purif Technol 78:33–41
Kang G-D, Cao Y-M (2012) Development of antifouling reverse osmosis membranes for water treatment: a review. Water Res 46:584–600
Khue VA (2014) Removal of copper and fluoride from wastewater by the coupling of electrocoagulation, fluidized bed and micro-electrolysis (EC/FB/ME) process. American Journal of Chemical Engineering 2:86
Kocharekar AR, Thakkar NV (2004) Extractive spectrophotometric determination of copper (II) and its applications in pharmaceutical samples and alloys. Hum Reprod 63:283–286
Li Y, Zeng X, Liu Y, Yan S, Hu Z, Ni Y (2003) Study on the treatment of copper-electroplating wastewater by chemical trapping and flocculation. Separation & Purification Technology 31:91–95
Liu G, Tao L, Liu X, Hou J, Wang A, Li R (2013) Heavy metal speciation and pollution of agricultural soils along Jishui River in non-ferrous metal mine area in Jiangxi Province, China. J Geochem Explor 132:156–163
Lou J-C, Huang Y-J, Han J-Y (2009) Treatment of printed circuit board industrial wastewater by ferrite process combined with Fenton method. J Hazard Mater 170:620–626
Luo F, Xu X, Li X, Qiu M, Wang P, Chen N (2011) Micro-electrolysis treatment of copper smelting of heavy metals in wastewater. Technology of Water Treatment 37:100–104
Masud J, Swesi AT, Liyanage WPR, Nath M (2016) Cobalt selenide nanostructures: an efficient bifunctional catalyst with high current density at low coverage. ACS Appl Mater Interfaces 8:17292
Miranda JM, Cobo A, Otero E, González JA (2007) Limitations and advantages of electrochemical chloride removal in corroded reinforced concrete structures. Cement & Concrete Research 37:596–603
Miyahara M, Hashimoto K, Watanabe K (2013) Use of cassette-electrode microbial fuel cell for wastewater treatment. J Biosci Bioeng 115:176
Orhan G, Gurmen S, Timur S (2004) The behavior of organic components in copper recovery from electroless plating bath effluents using 3D electrode systems. J Hazard Mater 112:261–267
Padervand M, Gholami MR (2013) Removal of toxic heavy metal ions from waste water by functionalized magnetic core-zeolitic shell nanocomposites as adsorbents. Environ Sci Pollut Res 20:3900–3909
Prakash N, Sudha PN, Renganathan NG (2012) Copper and cadmium removal from synthetic industrial wastewater using chitosan and nylon 6. Environ Sci Pollut Res 19:2930–2941
Prakash N, Latha S, Sudha PN, Renganathan NG (2013) Influence of clay on the adsorption of heavy metals like copper and cadmium on chitosan. Environ Sci Pollut Res 20:925–938
Prakash N, Latha S, Sudha PN, Renganathan NG (2016) Kinetics of removal of chromium from wastewater using chitosan based binary polymer blends. Synthesis and Reactivity in Inorganic, Metal-Organic, and Nano-Metal Chemistry 46:1310–1317
Rastmanesh F, Moore F, Kopaei MK, Keshavarzi B, Behrouz M (2011) Heavy metal enrichment of soil in Sarcheshmeh copper complex, Kerman, Iran. Environmental Earth Sciences 62:329–336
Reyes M, Patiño F, Tavera FJ, Escudero R, Rivera I, Pérez M (2009) Kinetics and recovery of xanthate-copper compounds by ion flotation techniques. J Mex Chem Soc 53:15–22
Sag Y, Kutsal T (1995) Biosorption of heavy metals by Zoogloea ramigera : use of adsorption isotherms and a comparison of biosorption characteristics. Chemical Engineering Journal & the Biochemical Engineering Journal 60:181–188
Salmani MH, Ehrampoush MH, Sheikhalishahi S, Dehvari M (2012) Removing copper from contaminated water using activated carbon sorbent by continuous flow. Journal of Community Health Research 1(1):11–18
She HB, Zhang TA (2010) Circulating economics and energy saving and discharge reducing of electrolysis aluminium industry promoted by science and technology. Light Metals 49:3–6
Tao FF, Zheng XU (2008) Electrochemical fabrication of dendrite-like copper crystals. J Mater Eng 39:150–153
Tian Y, Li Z, Xu H, Yang F (2008) Comparison on electro-reduction of Cu(II) using polypyrrole and stainless steel electrodes. Sep Purif Technol 63:334–340
Uğurlu M, Gürses A, Doğar Ç, Yalçın M (2008) The removal of lignin and phenol from paper mill effluents by electrocoagulation. J Environ Manag 87:420–428
Valenzuela F, Fonseca C, Basualto C, Correa O, Tapia C, Sapag J (2005) Removal of copper ions from a waste mine water by a liquid emulsion membrane method. Miner Eng 18:33–40
Wang YL, Sun Y (2013) Three-dimensional electrode used for wastewater containing Cu2+ from PCB factory. Adv Mater Res 864-867:1574–1577
Yang X (2009) Interior microelectrolysis oxidation of polyester wastewater and its treatment technology. J Hazard Mater 169:480–485
Zhang DX, Xu H, Liao YZ, Li HS, Yang XJ (2009a) Synthesis and characterisation of nano-composite copper oxalate powders by a surfactant-free stripping–precipitation process. Powder Technol 189:404–408
Zhang QB, Hua YX, Wang YT, Lu HJ, Zhang XY (2009b) Effects of ionic liquid additive [BMIM]HSO4 on copper electro-deposition from acidic sulfate electrolyte. Hydrometallurgy 98:291–297
Zhang YX, Jiang A, Cui T (2011) The research of the novel heavy metal chelating agent in the treatment of acid copper-containing wastewater. Guangdong Agricultural Sciences 38:124–126
Zhu W, Wang L, Liu S, Wang Z (2008) Characterization and catalytic behavior of silica-supported copper catalysts prepared by impregnation and ion-exchange methods. React Kinet Mech Catal 93:93–99
Acknowledgements
This work is jointly funded by the two National Natural Science Foundations of China (21476066) and (51271074).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Angeles Blanco
Rights and permissions
About this article
Cite this article
Xu, T., Lei, X., Sun, B. et al. Highly efficient and energy-conserved flocculation of copper in wastewater by pulse-alternating current. Environ Sci Pollut Res 24, 20577–20586 (2017). https://doi.org/10.1007/s11356-017-9280-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-017-9280-2