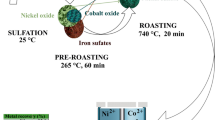
X-ray fluorescence analysis, X-ray powder diffraction, electron microprobe analysis, scanning electron microscopy, and thermal analysis are used to evaluate the material composition, structure and thermal properties of Dergamysh ore deposit and condensed roasted (750–850°C) products. Ore heating in air is accompanied by oxidation of sulfur and iron oxides, combustion and decomposition of sulfides, carbonate thermolysis, and decomposition of iron, copper, and zinc sulfates. Processes are generally completed up to 850°C. With desulfurization to the level of 60–94% sulfur the ore retains a porphyroblastic structure. Crystals (1–5 μm) of hexagonal and monoclinic pyrrhotites, corresponding to the general formula Fe0.80−0.93S, sphalerite and bornite border a mixture of fine (1 μm) olivine and spinel. In this case sulfides and oxides in contact with them achieve a similar concentration of non-ferrous metals, wt.%: up to 0.7 Co, 1.5–21.8 Cu, and 1.1–56.6 Zn. Heating a mixture of roasted (70–80% desulfurization) sulfide copper ore with nickeliferrous saprolite ore, calcium oxide and carbon in a weight ratio of 60:100:10:2.5 provides matte (4.1 wt. % Ni, 2.5 wt. % Cu, 0.38 wt. % Co, 2.1 g/ton Au, and 3.6 g/ton Ag) concentrating 90.3% nickel, 82.7% copper, 85.3% cobalt, and up to 99.0% of precious metals. The products of oxidation roasting of sulfide copper ores can be considered as an effective sulfiding agent and collector of valuable metals during smelting of nickeliferrous saprolite ores. The technology of joint reduction and sulfiding smelting of roasted copper ores and calcined saprolite ores is a promising way to use poor and mineral raw materials that are hard to enrich and to reduce SO2 emissions into the atmosphere.






Similar content being viewed by others
References
J. P. T. Kapusta, “JOM world nonferrous smelters survey. Part I: Copper (industrial survey),” JOM,56, No. 7, 21–27 (2004).
Technical Information Reference for the Best Available Technology TIS 3-2015. Copper Production, NDT Bureau (2015).
M. V. Knyazev, A. G. Ryabko, L. B. Tsymbulov, et al., “Two-zone Vanyukov furnace: New potential copper and nickel production,” in: Proc. оf the Sohn Int. Symposium (San Diego, USA. 2006), 8 (2006), 327–334.
M. L. Bakker, S. Nikolic, and P. J. Mackey, “ISASMELTTM TSL – Application for Nickel,” Miner. Eng., No. 24, 610–619 (2011).
F. Crundwell, Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals, Elsevier (2011).
E. N. Selivanov, R. I. Gulaeva, and A. M. Klyushnikov, “Study of the structure and phase composition of copper-cobalt sulfide ores of the Dergamysh deposit,” Svet. Met., No. 3, 13–17 (2016).
E. N. Selivanov, R. I. Gulyaeva, and A. M. Klyushnikov, “Technical and economic evaluation of direct metallurgical processing of sulfide ores,” Svet. Met., No. 3, 15–21 (2015).
E. N. Selivanov, A. M. Klyushnikov, R. I. Gulyaeva, et al., “Prospects of direct metallurgical treatment of sulfide ores,” in: Proc. Sci.-Pract. Conf. with Internat. Participation and Elements of School of Young Scientists “Prospects for Developing Metallurgy and Engineering Using Improved Fundamental Research and NIOKR”, Ural. Rabochii, Ekaterinburg (2015).
S. P. Nagaeva, O. P. Mezentseva, and M. V. Kozorez, “Mineralogical study of copper-cobalt containing ores of the Dergamysh deposit,” Gorn. Zh., No. 11, 31–35 (2014).
Yu. A. Savinova, L. Sh. Tsemekhman, and V. A. Popov, “Comparative analysis of substance composition of solid products of oxidation firing of sulfide ores concentrates of nonferrous metals,” Tsvet. Met., No. 11, 27–35 (2018).
Fang Qinfang, Zhang Hongwei, and Guo Ying, “Thermal decomposition of dolomite,” Advanced Materials Research,177, 617–619 (2011).
V. A. Luganov and V. I. Shabalin, “Thermal dissociation of pyrite during processing of pyrite-containing raw materials,” Canad. Metallurg. Quarterly,33, No. 3, 169–174 (1994).
L. Charpentier and P. J. Masset, “Thermal decomposition of pyrite FeS2 under reducing conditions,” Mater. Sci. Forum,654–656, 2398–2401 (2010).
S. R. Gzogyan and E. A. Chanturiya, “Effect of thermal action on iron sulfide and oxide,” Gorn. Inform. Tekhn. Byul., No. 5, 63–69 (2010).
Y. H. Luo, D. Q. Zhu, J. Pan, and X. L. Zhou, “Thermal decomposition behaviour and kinetics of Xinjiang siderite ore,” Miner. Proc. and Extractive Metallurgy,125, No. 1, 17–25 (2016).
J. Guntner and J. Hammerschmidt, “Sulphating roasting of copper-cobalt concentrates,” J. of the Southern African Institute of Mining and Metallurgy,112, 455–460 (2012).
G. G. Chuyanov, Dewatering, Dust capture and Environmental Protection [in Russian], Nedra, Moscow (1987).
I. D. Reznik, G. P. Ermakov, and Ya. M. Shneerson, Nickel, in 3 Vol. [in Russian], Vol. 2, Nauk. Tekhnol., Moscow (2001).
N. I. Kopylov, M. P. Smirnov, and M. Z. Toguzov, Composition Diagrams of Systems in Heavy Nonferrous Metallurgy [in Russian], Metallurgiya, Moscow (1993).
A. G. Khoroshavin, Forsterite [in Russian], Teplotekhnika, Moscow (2004).
G. V. Samsonov and S. V. Drozdova, Sulfides [in Russian], Metallurgiya, Moscow (1972).
N. Belzile, Yu-Wey Chen, Mey-Fang Cai, and Yuerong Li, “A review on pyrrhotite oxidation,” J. of Geoch. Expl., No. 84, 65–76 (2004).
I. D. Reznik, S. I. Sobol’, and V. M. Khudyakov, Cobalt, in 2 Vol. [in Russian], Vol. 1, Mashinostroenie, Moscow (1995)
E. N. Selivanov, A. M. Klyushnikov, V. M. Chumarev, and R. I. Julyaeva, RF Patent 2657267, МPК С22В 23/02, С22В 5/08. Charge for Oxidation-Sulfiding of an Oxidized Nickel Ore Melt, Claim 06.08.2017; Publ. 06.09.2018; Bull. No. 16.
P.-M. Guo and L.-F. Zhang, “Study on catalytic mechanism of Boudouard reaction,” Iron and Steel, No. 43, 26–30 (2008).
E. N. Selivanov, A. A. Sorokin, N. V. El’kina, et al., “Separation of impurity elements during shaft melting of Serov deposit ore,” Tsvet. Met., No. 10, 17–20 (1993).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 63, No. 8, pp. 83–90, August, 2019.
Rights and permissions
About this article
Cite this article
Selivanov, E.N., Klyushnikov, A.M. & Gulyaeva, R.I. Application of Sulfide Copper Ores Oxidizing Roasting Products as Sulfidizing Agent During Melting Nickel Raw Materials To Matte. Metallurgist 63, 867–877 (2019). https://doi.org/10.1007/s11015-019-00901-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-019-00901-z