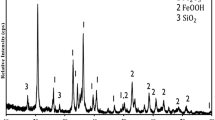
In the steel industry, ferromanganese is an important additive. During the production of ferromanganese the ore goes through prereduction and reduction. The prereduction step is very important, as the degree of reduction during ferromanganese is important. Although many investigations have been conducted on different manganese ores, the current study investigated different phases that formed during prereduction when South African ore was used. The ore was mixed with carbon in excess as the reducing agent, and no fluxes were added while CO gas was blown in the furnace. The temperature was varied from 1000°C to 1200°C with 100°C interval. For each temperature, the time varied from 30 min to 90 min with 30 min interval. The ore to carbon ratio was 4:1. After the experiments, the resulting products were subjected to chemical and phase analysis using XRD, XRF and SEM.












Similar content being viewed by others
References
M. Kalenga, M. Tangstad, and X. Pan, “Manganese alloys production: impact of chemical compositions of raw materials on the energy and materials balance,” in: The Thirteenth International Ferroalloys Congr., June 9–12, Almaty, Kazhastan (2013), pp. 647–654.
V. Kivinen, H. Krogerus, and J. Daavittila, “Upgrading of Mn/Fe ratio of low-grade manganese ore for ferromanganese production,” in: The Twelfth International Ferroalloys Congr., Proceedings, June 6–9, Helsinki, Finland (2010), pp. 467–476
S. E. Olsen, M. Tangstad, and T. Lidstad, Production of Manganese Ferroalloys, 143–149 (2007), ISBN 978-82-519-2191-6.
S. Maroufi, G. Ciezki, S. Jahnashai, S. Sun, and O. Ostrovsky, “Dissolution of silica in slag in silicomanganese production,” in: The Fourteenth International Ferroalloys Congr., Proceedings, May 31–June 04, Kiev, Ukraine (2015), pp. 479–487.
J. Daavittila, H. Krogerus, P. Oikarinen, and R. Sarkkinen, “Sintered manganese ore and its use in ferromanganese production,” in: The Nineth International Ferroalloys Congr., Proceedings, 3–6 June, Quebec City, Canada (2001), pp. 212–222.
W. Ding and S. E. Olsen, “Manganese and silicon distribution between slag and metal in silicomanganes production,” ISIJ Inmternational, 40, No. 9, 850–856 (2000).
K. Manoj, S. Ranganathan, and S. N. Sinhal, “Kinetics of reduction of different manganese ores,” in: The Eleventh International Ferroalloys Congr., Proceedings, 18–21 February, New Dheli, India (2007), pp. 241–246.
M. Yastreboff, O. Ostrovski, and S. Ganguly, “Effect of gas composition on the carbothermic reduction of manganese oxide,” ISIJ International, 43, No. 2, 161–165 (2003).
T.-A. Skjervheim, Dr. Thesis, NTH, Trondheim, MI-Report, 26 (1994).
C. Theresa, J. Zietsman, and C. Pistorius, “Predicted effect of ore composition on slag formation in manganese ore reduction,” Mineral Processing and Extractive Metallurgy, Transactions of the Institutions of Mining and Metallurgy: Section C, 123, Issue 3, 1–7 (2014).
R. H. Eric and E. Burucu, “The mechanism and kinetics of the carbothermic reduction of Mamatwan manganese ore fines,” Miner. Eng., 5, No. 7, 795–815 (1992).
G. Akdogan and R. H. Eric, “Kinetics of the solid-state carbothermic reduction of Wessel manganese ores,” Metall. Mater. Trans. B, 26, 13–24 (1995).
J. S. J. Van Deventer, “The effect of gangue components on the reduction of manganosite by graphite: an isothermal kinetic study,” Thermochim. Acta, 112, 365–377 (1987).
R. Kononov, O. Ostovski, and S. Ganguly, “Carbothermic solid state reduction of manganese ores: 3. Phase development,” ISIJ Int., 49, No. 8, 1115–1122 (2009).
Acknowledgment
A special recognition to Prof. Merete from NTNU/ Norway and Dr. Pan from the University of Johannesburg for their great support and guidance in this research. We also thank the South African manganese industry for their support in providing samples, and the laboratory technicians of the University of Johannesburg in the Department of Metallurgy for their unconditional support in the laboratory and their availability.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Metallurg, Vol. 62, No. 11, pp. 20–26, November, 2018.
Rights and permissions
About this article
Cite this article
Kalenga, M., Tangstad, M. & Pan, X. Phase Relations in Ferromanganese Production During Prereduction: South African Ores. Metallurgist 62, 1100–1114 (2019). https://doi.org/10.1007/s11015-019-00762-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11015-019-00762-6