Abstract
The traditional ironmaking technologies (including coking, sintering, pelletizing, and BF ironmaking process) are carbon-intensive, which makes the industry a significant contributor to global CO2 emissions. Hydrogen replacement of carbon in steelmaking processes is a sustainable way to reduce CO2 emissions. First, the reduction thermodynamics and kinetics of iron oxide by carbon and hydrogen are compared. Then, the latest researches on different hydrogen reduction technologies in ironmaking industry are compared and analyzed. Based on this, the advantages and problems faced by hydrogen-based reduction over carbon-based reduction are presented. And finally, the possible pathways for the future development of hydrogen metallurgy are proposed, hoping to provide guidance for the hydrogen metallurgy in the steel industry. The reduction product of hydrogen metallurgy is H2O, and has a faster reduction rate than CO reduction. Therefore, hydrogen metallurgy is considered to be an effective way to achieve low-carbon green transformation in the metallurgical industry.
Graphical Abstract

Similar content being viewed by others
Introduction
The rapid development of industrialization has led to the intensification of global warming. Global greenhouse gas (GHG) concentrations reached new highs in 2020, with concentrations of CO2, CH4 and NOx higher than pre-industrial levels, 149%, 262%, and 123% respectively [1,2,3]. As the most important GHG, CO2 has become the focus of energy conservation and emission reduction [4,5,6]. In 2021, global CO2 emissions increased by 6% to 36.3 billion tons, of which energy consumption emissions account for up to 73.2%, including electricity, heat, and transportation [7, 8]. The CO2 emissions of the steel industry account for about 6.7% of the global rates, and a ton of steel produced from virgin ore emits 1.8 t CO2 [9, 10]. Figure 1 shows the distribution of world steel production, and the proportion of energy consumption and carbon emissions of the steelmaking process in China. In 2021, the global crude steel output was 1.95 billion tons, with a growth rate of 3.7% and China accounted for more than 50% with 11.9 billion tons of CO2, accounting for 33% of the global total, making it the world's largest carbon emitter [11, 12]. About 80% of the CO2 emissions from the steel industry come from the ironmaking system. Blast furnace (BF) ironmaking consumes large quantities of coal as fuel and reductant, resulting in more than 70% of CO2 emissions from the steel industry [13, 14]. The carbon emissions of the ironmaking system (including coking, sintering, pelletizing, and BF ironmaking process) accounted for 82.79% of the total emissions of the long process of iron and steel enterprises, of which BF ironmaking accounted for 67.02%, sintering accounted for 8.54%, and coking accounted for 6.13% [15]. As a result, the focus of decarbonization in the steel industry is on integrated plants using BFs [16, 17].
There are various methods of ironmaking including BF, direct reduction, smelting reduction, and more. The basic principle behind all these methods is to reduce the ore into metallic iron (including hot metal, pig iron and sponge iron/direct reduced iron (DRI)) by subjecting it to a specific atmosphere of reducing substances such as CO, H2, C, and a suitable temperature. BF ironmaking is a process that involves heating a mixture of iron ore, oil, coal, coke, and other raw materials in a BF to extract oxygen from iron oxide and produce hot metal. Direct reduction ironmaking is a process that involves reducing iron ore to obtain metallic iron in a reaction vessel using gas or solid reducing agents at a temperature below the melting point of iron. The smelting reduction ironmaking process, on the other hand, uses non-coking coal as the energy source. In this process, the iron oxide reduction occurs in the high-temperature melting state, and molten iron is produced after complete separation from slag iron. Table 1 displays the benefits and drawbacks of different iron-making techniques. In 2022, 426 steel enterprises in China emitted 423,800 tons of particulate matter, 152,200 tons of SO2, and 352,900 tons of NOx. This represents a 6.05% decrease in particulate matter emissions, and a 17.55% and 13.71% decrease in SO2 and NOx emissions, respectively, compared to enterprises of the same size in 2021 [18]. These data establish that to decarbonize the steel industry in China, major transformations in ironmaking methods are essential [19].
As an emerging strategic clean reductant and energy carrier, hydrogen has the characteristics of abundant sources, high thermal efficiency, and high energy density [20]. It is also portable, storable, and potentially renewable, which plays an important role in the future global energy structure change [21]. The carbon reduction strategies are being phased in based on how readily they can be implemented, the cost, and the availability of the infrastructure and resources needed. Energy conservation is considered the foremost measure, followed by the partial replacement of coal with biomass or hydrogen, the developing of a hydrogen-rich BF technology, and the expansion of hydrogen-based non-BF direct/smelting reduction processes [22, 23]. The third strategy is a complete abandonment of coal by using hydrogen as the only reductant in shaft or fluidized bed reactors. Generated by renewable or nuclear electricity, hydrogen-based ironmaking is emerging as the ultimate solution towards zero-carbon steel [24].
Hydrogen energy is one of the cleanest, low-carbon sources. It has become an important topic for low-carbon steel production, where hydrogen is used to replace carbon. Currently, hydrogen-based steelmaking concepts include hydrogen-rich BF ironmaking technology [25, 26], hydrogen-based direct reduction process [27, 28], hydrogen-based smelting reduction process [29], and hydrogen-based plasma direct steelmaking process [30, 31]. This paper will review the theoretical feasibility of hydrogen metallurgy, and the typical hydrogen metallurgy ironmaking processes in the world will be compared. The opportunities and challenges for the development of hydrogen metallurgy will also be discussed. Finally, the development direction of hydrogen-based ironmaking technology prospected. It is aimed at providing a reference for accelerating the research and development of hydrogen metallurgy and promoting the low-carbon process of the global iron and steel industry.
Reduction Behavior of Iron Oxides by Carbon and Hydrogen
The process of reducing iron oxide using carbon-based or hydrogen-based reductants is complex. Although the reaction mechanism is relatively simple, the reduction reaction process undergoes complicated changes due to variations in process parameters, reaction environment, and reactant types. By conducting a thermodynamic and kinetic analysis of the reduction reaction process, it is possible to design a more effective hydrogen reduction reaction process, leading to improved economic benefits.
Thermodynamic Considerations
The smelting process is to obtain hot metal with the required temperature and composition from iron ore economically. Under the condition of the lowest possible energy consumption, the processes of reduction, slagging, heat transfer, and slag-iron reaction are efficiently completed through the controlled countercurrent flow of the charge and gas [32, 33]. Carbon metallurgy involves the conversion of solid carbon (coal, coke, etc.) into CO under incomplete combustion conditions for a reduction reaction, while gaseous hydrogen directly participates in the reduction reaction without any conversion. Carbon metallurgy not only produces a large amount of CO2, but also releases other pollutants such as SOx, NOx, and dioxins which are associated with the combustion of coal/coke. On the contrary, the reduction of hydrogen to produce H2O can coexist in harmony with nature [34].
There are two important temperatures for H2 and CO to reduce iron oxides, which are 570 °C and 820 °C, respectively, as shown in Fig. 2. FeO does not exist below 570 ℃, and Fe3O4 is directly reduced to metallic iron. Reduction by CO is an exothermic reaction, while H2 reduction is endothermic. Above 570 ℃, the first step of reduction (Fe2O3-Fe3O4) requires a weak reducing atmosphere and can be regarded as an irreversible reaction [35]. For the second step (Fe3O4-FeO), the reduction by both CO and H2 is endothermic. The third step (FeO-Fe), being the hardest of the reactions, requires strong reducing conditions. Reduction of wustite by H2 is still an endothermic reaction, whereas that by CO is exothermic [36, 37]. Above 820 ℃, the reducing potential of H2 is higher than that of CO, thanks to its better kinetic behavior and the water–gas shift reaction, Reaction (9) in Table 2. Unless the produced gases in the reduction process are forced out of the system, the reactions can come to a stop due to the increased concentration of CO2 and H2O [38, 39]. Water vapor, in particular, is believed to be readily adsorbed to the surface of oxides, blocking the access of the reducing gas, and slowing the reactions [40, 41].
Table 2 shows the thermodynamic parameters of the iron oxides reduction, Gibbs free energy (\({\Delta }_{\mathrm{r}}{G}_{\mathrm{m}}^{\Theta }\)) and equilibrium constant (K) values [35, 42]. Comparing the thermal effects of reducing iron oxides, the energy consumption by CO reduction is smaller than that of H2, giving CO an energy advantage. Overall, a switch from CO to H2 would result in an increased energy demand of ~ 900 kJ/kg Fe.
Kinetics Considerations
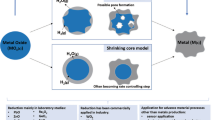
The existing models to describe the gas–solid reaction mainly include the adsorption autocatalysis theory, the solid-phase diffusion theory, and the unreacted core model theory [43,44,45]. Kinetic studies on the reduction of iron oxides by CO and H2 are typically described by the unreacted core model, depicted in Fig. 3. In this model, particles of iron oxide, packed in a compact, are reduced on contact with gas, leaving a reaction product on the surface that moves inwards with the progression of the reduction.
From a kinetic point of view, the reduction of iron oxides with H2 is faster than that with CO. Zuo et al. [46] studied the reduction behavior of hematite pellets in different gas mixtures at 1000 °C. The effective diffusion coefficients were determined based on the unreacted core model, including internal reduction, pore diffusion, and gas-film mass transfer. The results are shown in Fig. 4 in which t1 represents the total reduction time when the reduction rate is controlled only by external diffusion, and F represents the ratio of time to total reaction time. In the presence of CO, the effective diffusion coefficient decreases sharply. In general, H2 always has a higher rate of reduction than CO, especially between 700 and 900 °C. However, at higher temperatures, the smaller the difference in reduction rates because of the higher activation energy of reduction by hydrogen[47].
a Change of reduction degree with reducing time under different CO/H2 at 1000 ℃; b relationship of (t − t1)/F and 3F − 2F2 [34]
Gas diffusivity depends on the temperature and physical properties of the gas mixture and diffusing species such as viscosity, molecular size, and pressure[48,49,50]. The higher the temperature, the faster the movement of gas molecules. Compared with CO, hydrogen has a lower viscosity and smaller molecule, hence a higher diffusion coefficient. As a result, the gas-phase rate of mass transfer for hydrogen is estimated to be 5–6 times greater than that of CO [51, 52]. The reaction rate constants for H2 and CO at 800 ~ 900 °C are 0.01 ~ 0.02 s−1, and 0.001 ~ 0.002 s−1, respectively [51]. The effective diffusion coefficient of H2 decreases sharply in the presence of CO.
Technological Status of Hydrogen Reduction in Steel Industry
Hydrogen metallurgy is a concept that was introduced in the last century, which replaces carbon with hydrogen to reduce iron ore. This innovative method of steel production completely eliminates pollutants and CO2 emissions from the source, and is considered the most important approach to achieving zero carbon emissions. Worldwide, the popularity of hydrogen metallurgy processes based on shaft furnaces and the use of hydrogen-rich smelting technology based on BFs as follows is increasing, as they are clean and revolutionary steel production techniques.
Hydrogen-Based Reduction Technologies Development
CO2 Ultimate Reduction in Steelmaking Process for Cool Earth 50 (COURSE50) in Japan
Carbon dioxide from the steel industry in Japan accounted for 12.4% of the country’s emissions in 2020. As one of the longest-running decarbonization programs, Japan has launched an environment-harmonious ironmaking, COURSE50, to achieve more effective CO2 emission reduction [53, 54]. The outline of CO2 ultimate reduction in steelmaking by the COURSE50 Project is shown in Fig. 5. The research is mainly focused on two areas: 1) Direct reduction of iron ore with hydrogen along improvements in the conventional BF route, aiming to achieve 10% CO2 reduction. Some proposed technologies include the use of hydrogen to directly reduce iron ore, coke oven gas upgrading technology to increase hydrogen content, production of high-strength and high-reactivity coke; 2) CO2 separation and recovery from the BF gas, to reduce CO2 emissions by 20%.
Comparison of conventional blast furnace, COURSE50, and super COURSE50 Program [37]
POSCO Hydrogen Reduction Ironmaking Process in Korea
POSCO started to develop the VHTR (Very High-Temperature Reactor) and SMART (System-integrated Modular Advanced Reactor) in May 2010 [55, 56]. Four technical routes are used to reduce CO2 emissions by more than 15%. (1) Hydrogen Amplification Technology: the hydrogen content in coke oven gas is increased after modification to meet the requirements of BF hydrogen-based reduction. (2) Improving the existing operations by H2 injection into BF. The coke oven gas (COG) and natural gas produced in the coke production process are used, and H2 is extracted to control temperature and prevent oxidation in the steel production process. The proportion of lower-grade pellets can be increased to promote the reduction of iron ore. What’s more, the full use of recyclable scrap, the application of CCUS technology, the use of pure oxygen instead of the traditional preheating air, the use of magnesium alkaline pellets, and highly reactive iron coke are feasible ways to reduce coke[57]. (3) Development of materials for handling hydrogen such as ultra-heat-resistant and ultra-corrosion-resistant raw materials: It is necessary to first develop ultra-corrosion-resistant high-temperature materials that can be used in the delivery and storage of high-temperature, high-pressure hydrogen, and (4) Hydrogen based production of DRI followed by its melting in electric furnaces replacing high-grade scrap. POSCO is currently operating the FINEX facility, which uses a reduction gas containing 25% hydrogen and is developing a new hydrogen reduction model, Hydrogen-based Steelmaking (HyREX) to make DRI. Figure 6 shows the comparison of blast furnaces, FINEX, HyREX, and other shaft furnace direct reduction processes [58].
Comparison of blast furnace, FINEX, HyREX, and other shaft furnace direct reduction processes [40]
ThyssenKrupp Hydrogen-Based Ironmaking Project in Germany
ThyssenKrupp has launched tests into the use of hydrogen and green energy to produce steel in November 2019, branded as "BF 2.0" [59,60,61]. Figure 7 shows the hydrogen metallurgy development roadmap of ThyssenKrupp. ThyssenKrupp plans to build a direct reduced iron plant in Duisburg for completion in 2025 with an annual capacity of 1.2 million tons. The target reduction in the emissions is 30% by 2030. The technical route of hydrogen metallurgy is first to inject hydrogen into BF, and then gradually replace BF with direct reduction (DR) technology. Steel mill exhaust gases contain valuable chemical raw materials such as carbon in the form of CO and CO2, as well as N and H. In ThyssenKrupp’s program, these will be used to produce synthesis gas as well as chemicals such as ammonia, methanol, polymers, and higher alcohols.
Baosteel Hydrogen Based Low-Carbon Technology Route in China
In February 2022, Baosteel’s Zhanjiang Iron and Steel Zero-Carbon Demonstration Plant officially started to be built. The plant is designed for a million-ton DRI with a total investment of RMB 1.89 billion for the hydrogen-based shaft furnace [62]. The carbon–neutral steelmaking roadmap of Baowu Steel has four major elements related to ironmaking: extreme energy efficiency, hydrogen-rich carbon cycle BF, hydrogen-based shaft furnace, and metallurgical resource recycling [63]. The hydrogen-rich carbon cycle BF technology separates CO2 from the gas, turns the by-product gas into a gas with high reduction potential, and reuses it in the BF to fully utilize the carbon chemical energy. Figure 8 shows Baowu’s hydrogen-based low-carbon program [64].
SSAB Hydrogen Ironmaking Technology in Swedish
In August 2020, the world's first pilot plant for fossil-free production of sponge iron (DRI/HBI) was launched in Luleå, Sweden. The Breakthrough Hydrogen Ironmaking Technology (HYBRIT) project is a collaboration between Swedish Steel Corporation (SSAB), LKAB Mining Company and Vattenfall, and represents a decisive step towards achieving fossil-free steelmaking. Figure 9 shows the comparison of some emissions and consumptions for the SSAB’s BF route and the HYBRIT process [65]. The plant is scheduled to complete testing by 2024 and plans to establish a near-zero carbon emissions steel commercial plant by 2035. The HYBRIT project will test multiple stages of a process for the direct reduction of iron ore using hydrogen, which will be produced by the electrolysis of water. The HYBRIT route has a much lower CO2 emissions due to the almost complete reliance on hydrogen use in the shaft furnace DR processes. The use of biomass in this process is also being investigated. The current disadvantage of the process is that it costs 1.2 ~ 1.3 times as much as a BF [66].
Comparison of some emissions and consumptions for the SSAB BF route and the HYBRIT concept [47]
Other Hydrogen Metallurgy Explorations
Along with the "low-carbon economy" development across the world, to significantly reduce the overall carbon emissions in steel production, countries are rushing to carry out some promising hydrogen metallurgical projects. In the context of the "Carbon neutrality" goals, low-carbon metallurgy and hydrogen metallurgy have become hot topics among steel enterprises and research institutions. International steel companies in different regions have already started experimenting and exploring low-carbon metallurgical processes and hydrogen metallurgy technologies. The existing low-carbon hydrogen metallurgical ironmaking methods in each region are shown in Fig. 10. With the depletion of traditional resources like coal and the support of national policies, the prospects for the development of low-carbon ironmaking technologies are promising.
Dillingen and Saar Steel in Germany are studying the injection of hydrogen-rich coke oven gas in BF [60]. The ArcelorMittal Smart Carbon Concept proposes a carbon–neutral route, including the use of clean energy, recycled carbon, and carbon capture/storage, while exploring hydrogen direct reduction of iron ore at the same time [67, 68]. The GFG Alliance is promoting the development of hydrogen reduction technology based on natural gas with the ultimate goal of achieving zero emissions [69]. Tata Steel will convert its steelmaking facilities from coal-based to hydrogen-based utilizing direct reduced ironmaking (DRI) technology, in which iron ore is directly reduced using natural gas, progressively increasing amounts of hydrogen, and subsequently melted and refined in large and efficient rectangular electric smelting furnaces [70]. Voestalpine has tested the application of green hydrogen in various steel production processes [71]. Hegang Group in China has cooperated with Italy’s Tenova Group to advance hydrogen production and hydrogen reduction technologies, planning to build the first 1.2-million-ton hydrogen metallurgy demonstration project. The hydrogen-based direct reduction iron project (CSDRI) was launched by Shanxi Zhongjin Technology Group, including coke oven gas conversion and purification technology, especially low-pressure deep desulfurization purification technology [72].
Overall, the existing hydrogen metallurgy that has been applied in production is divided into blast furnace hydrogen-rich smelting and shaft furnace hydrogen metallurgy low-carbon metallurgy technology. In the coming decades, the BF ironmaking process, featuring mature technology, large production capacity, and high efficiency, will continue to be the mainstream ironmaking equipment that supports the huge demand for steel materials in the world. Hydrogen-rich low-carbon smelting in BFs is the primary path to achieve low-carbon production in the steel industry on a large scale [73]. Table 3 compares the world's major hydrogen-based BF process applications. On the other hand, shaft furnace hydrogen metallurgy is a key technology for achieving low-carbon metallurgy, Table 4 compares the typical hydrogen-based direct reduction shaft furnace process [48].
Opportunities and Challenges for Hydrogen Metallurgy in Steel Industry
The reduction of carbon emissions is crucial, and addressing the energy problem is the key to achieving this goal. To achieve a low-carbon transformation, the steel industry needs to adopt an approach that involves adjusting its energy structure by replacing carbon with hydrogen. The reform of the hydrogen metallurgy process has attracted attention throughout the entire hydrogen energy industry chain, leading to breakthroughs in hydrogen production, storage, and transportation technologies. It is necessary to figure out the advantages and challenges for developing new advanced hydrogen-based metallurgy technologies.
Advantages of Hydrogen Metallurgy
Figure 11 shows the flow chart of hydrogen-based low-carbon iron and steel metallurgy. From an energy perspective, the reduction of iron oxides with hydrogen can reduce carbon consumption, and reduce the direct reduction ratio. The purity of molten iron can be improved by reducing the contamination from the fuels (coke, coal), resulting in a consistent chemical composition of the molten iron. Besides, replacing C with H can reduce fuel ratio, reduce slag volume and flux consumption. Through kinetic behavior analysis, H2 reduces iron oxides faster than CO, which can improve the reduction rate and productivity, and accelerate the indirect reduction when use in BF. Further, with hydrogen, the control of the carbon content of the steel is easier, with minimal need for decarbonization processes; hence an expected lower capital and operating costs associated with steelmaking.
In future hydrogen-based steelmaking plants, the operating and capital costs will differ from traditional plants. Sintering, coking, and their associated facilities will become obsolete, while the production and handling of hydrogen will become crucial. The design and operating conditions of H2-reactors will differ from those of BFs, leading to significant changes in steelmaking processes. This includes reconsideration of carbon control, slag amount and chemistry, as well as heat supply (electric heating). The impacts of these on the economics are still not fully analyzed or reported.
For the BF, injecting hydrogen to replace part of the pulverized coal can improve the hearth conditions and reduce the generation of unburned pulverized coal [81]. The gas permeability of the charge can be improved, which may increase the onset melting temperature of the charge, and lower the position of softening and melting zones. Specifically, when using the method of injecting coke oven gas for hydrogen-rich smelting, the concentration of reducing gas in the furnace increases, which accelerates the reduction rate of the furnace charge. This technological improvement has resulted in a ~ 15% reduction in coke ratio and a ~ 9% reduction in carbon emissions.
Challenges in Hydrogen Metallurgy
The agglomeration or adhesion of iron ore particles in the process of hydrogen-based direct reduction is an important factor affecting production efficiency. In the local overheating area, the mixed minerals with low melting points will soften and bond together, and a solid reaction between gangue and FeO produces liquid phase resulting in bonding. Therefore, hydrogen-based direct reduction requires higher ore grade and as low gangue content as possible. With the gradual mining of iron ore, high-grade iron ore is scarce, making it difficult to support large-scale DRI production development. Research and advancements are needed to enable the treatment of low-grade iron ores in DR processes and subsequent steel refining.
In addition, a hydrogen economy and infrastructure are far from maturity to support the significant quantities required for an industry as large as steelmaking. The current hydrogen production is almost entirely based on the use of fossil fuels as the primary source of energy, which defeats the purpose of moving to low-carbon technologies [82]. The cost of green hydrogen from electrolysis of water is 2 to 3 times that of coke oven gas and reformed natural gas. Iron and steel enterprises have the foundation for developing hydrogen metallurgy only if inexpensive and abundant hydrogen is available [80].
The next challenge for a hydrogen metallurgy scheme is storing, transporting, and utilizing gases rich in hydrogen. The liquefaction temperature of hydrogen is much lower than that of other gases, and it consumes a significant amount of energy[83]. The integrity of the gas handling facilities, materials compatible with hydrogen and water vapor at high temperatures and pressures, and safety measures around hydrogen use have to find robust solutions before hydrogen utilization becomes widespread.
Pathways for Hydrogen Metallurgy Development
The preparation and transportation of hydrogen is the foundation of the hydrogen metallurgy. Breaking through of low-cost green hydrogen preparation technology is an irresistible trend for the development of hydrogen metallurgy. Hydrogen is an environmentally friendly and efficient energy medium with significant potential in addressing energy and environmental problems [83]. Table 5 shows the comparison of technologies and indicators of different hydrogen production processes. Table 6 shows the comparative analysis of the advantages and disadvantages of hydrogen transportation technology path. The development of hydrogen production and transportation technology will greatly improve the feasibility of hydrogen metallurgy industrial production.
Hydrogen metallurgy technology should get the same support and encouragement as hydrogen energy. The breakthrough of hydro-based ironmaking technology is the top priority of gas development. It is mainly aimed at the existing hydrogen rich reduction technology of BF with hydrogen instead of carbon, the hydrogen base direct reduction process of obtaining solid reduced iron with hydrogen rich or whole hydrogen as reducing agent, and the hydrogen base smelting reduction process of producing hot metal with hydrogen or pure hydrogen gas as reducing agent. On the one hand, the theoretical and experimental research on the principle and process should be strengthen. And on the other hand, the technology should be gradually optimized and innovated in practical production. It mainly includes raw material treatment and control technology, process operation technology, product treatment and recycling technology, production safety and economy. In addition, it is necessary to increase efforts to develop new advanced hydrogen-based iron making technologies such as plasma hydrogen metallurgy.
Summary and Prospect
The steel industry is the main body responsible for achieving the goal of carbon neutrality, and hydrogen metallurgy is the general trend of future development. The future steel energy structure will achieve subversive adjustment, and the process technology will undergo fundamental changes. To usher in the “hydrogen metallurgy era”, the traditional carbon metallurgy production mode should be broken, reducing the proportion of carbon metallurgy. The ultimate goal of hydrogen metallurgy is to develop the process application of pure hydrogen as a reducing agent, completely get rid of the carbon-base fuels, and achieve “pure hydrogen metallurgy”.
In general, hydrogen is more suitable as a reducing agent, and carbon is more suitable as an energy medium. Some of the metallurgical challenges needing attention are the energy balance of reactors due to the endothermic nature of hydrogen reduction, the treatment of low-quality ores with hydrogen, and steelmaking processes when excess carbon may not be present in the liquid iron. The heat compensation in the process of pure hydrogen reduction is one of the key factors limiting the development of hydrogen metallurgy, so the combination mode of "hydrogen-carbon" efficient reduction and "electricity-carbon" heat supply is an important transition way.
The preparation and transportation of hydrogen is the foundation of the hydrogen metallurgy. There are more critical, non-metallurgical constraints that have to be addressed, including supplying the quantities of hydrogen required, cleanly producing the hydrogen with minimum reliance on fossil fuels, and lowering the cost of delivered hydrogen. Hydrogen metallurgy technology should get the same support and encouragement as hydrogen energy. The breakthrough of hydro-based ironmaking technology is the top priority of gas development. On the one hand, the theoretical and experimental research on the principle and process should be strengthen. And on the other hand, the technology should be gradually optimized and innovated in practical production.
References
United States Environmental Protection Agency. Overview of greenhouse gases. https://www.epa.gov/ghgemissions/overview-greenhouse-gases
Calise F, Cappiello FL, Cimmino L, Vicidomini M (2023) Dynamic modelling and energy, economic, and environmental analysis of a greenhouse supplied by renewable sources. Appl Sci 13:1
Sesana E, Gagnon AS, Ciantelli C, Cassar J, Hughes JJ (2021) Climate change impacts on cultural heritage: a literature review. Wiley Interdiscipl Rev 12:e710
Hickmann T, Widerberg O, Lederer M, Pattberg P (2021) The United Nations framework convention on climate change secretariat as an orchestrator in global climate policymaking. Int Rev Adm Sci 87(1):21–38
Verschuuren J (2022) Climate change adaptation under the United Nations Framework Convention on Climate Change and related documents Research handbook on climate change adaptation law. Edward Elgar Publishing, Cheltenham, pp 14–30
Miyamoto M, Takeuchi K (2019) Climate agreement and technology diffusion: impact of the Kyoto protocol on international patent applications for renewable energy technologies. Energy Policy 129:1331–1338
Lawrence MG, Schäfer S, Muri H, Scott V, Oschlies A, Vaughan NE et al (2018) Evaluating climate geoengineering proposals in the context of the Paris agreement temperature goals. Nat Commun 9(1):1–19
Rogelj J, Huppmann D, Krey V, Riahi K, Clarke L, Gidden M et al (2019) A new scenario logic for the Paris agreement long-term temperature goal. Nature 573(7774):357–363
Hall BD, Crotwell AM, Kitzis DR, Mefford T, Miller BR, Schibig MF et al (2021) Revision of the World Meteorological Organization global atmosphere watch (WMO/GAW) CO2 calibration scale. Atmos Meas Tech 14(4):3015–3032
Lei R, Sheng Z, Xunmin O (2023) The carbon reduction potential of hydrogen in the low carbon transition of the iron and steel industry: the case of China. Renew Sustain Energy Rev 171(1):1130261–1130317
International Energy Agency (2020) Hydrogen. https://www.iea.org/reports/iron-and-steel-technology-roadmap
Sun M, Pang K, Barati M, Meng X (2023) Development and problems of fluidized bed ironmaking process: an overview. J Sustain Metall. https://doi.org/10.1007/s40831-023-00746-6
Gu W, Zhao X, Yan X, Wang C, Li Q (2019) Energy technological progress, energy consumption, and CO2 emissions: empirical evidence from China. J Clean Prod 236:117666
Lei W, Xie Y, Hafeez M, Ullah S (2022) Assessing the dynamic linkage between energy efficiency, renewable energy consumption, and CO2 emissions in China. Environ Sci Pollut Res 29(13):19540–19552
Choi J, Kim W, Choi S (2022) The economic effect of the steel industry on sustainable growth in China—a focus on input–output analysis. Sustainability 14:4110
Wulf C, Linssen J, Zapp P (2019) Review of power-to-gas projects in Europe. 12th international renewable energy storage conference: IRES 2018, Dusseldorf, Germany, 13–15 March 2018, 367–378
Rechberger K, Andreas Sasiain C, Wolfmeir A, Harris H (2020) Green hydrogen-based direct reduction for low-carbon steelmaking. Steel Res Int 91(11):202000110
(2023) 2022 Steel Industry Green Development Level Assessment Report by Environmental Engineering Evaluation Center of the Ministry of Ecology and Environment
Yu X, Tan C (2022) China’s pathway to carbon neutrality for the iron and steel industry. Glob Environ Chang 76:102574
Qazi UY (2022) Future of hydrogen as an alternative fuel for next-generation industrial applications. Challenges Expect Opportunities Energies 15(13):4741
Dutta S (2014) A review on production, storage of hydrogen and its utilization as an energy resource. J Ind Eng Chem 20(4):1148–1156
Bailera M, Lisbona P, Pea B, Romeo LM (2021) A review on CO2 mitigation in the iron and steel industry through power to X processes. J CO2 Util 46:101456
As D, Sabrina G, Sven L, Gilles K, Anand A, Miriam V et al (2021) Reduction of CO2 emission from off-gases of steel industry by dry reforming of methane. Angew Chem 60(21):11852–11857
Zhidong T, Qi Z, Yongsheng S, Peng G, Yuexin H (2022) Prospects of green extraction of iron from waste dumped flotation tailings by H2: A pilot case study. J Clean Prod 330(1):1298531–1298612
Chen Y, Zuo H (2021) Review of hydrogen-rich ironmaking technology in blast furnace. Ironmaking Steelmaking 48(6):749–768
Zhang C, Vladislav L, Xu R, Sergey G, Jiao K, Zhang J et al (2022) Blast furnace hydrogen-rich metallurgy-research on efficiency injection of natural gas and pulverized coal. Fuel 311:122412
Rechberger K, Spanlang A, Sasiain Conde A, Wolfmeir H, Harris C (2020) Green hydrogen-based direct reduction for low-carbon steelmaking. Steel Res Int 91:2000110
Wang R, Zhao Y, Babich A, Senk D, Fan X (2021) Hydrogen direct reduction (H-DR) in steel industry—an overview of challenges and opportunities. J Clean Prod 329:129797
Liu W, Zuo H, Wang J, Xue Q, Ren B, Yang F (2021) The production and application of hydrogen in steel industry. Int J Hydrogen Energy 46(17):10548–10569
Badr K (2007) Smelting of iron oxides using hydrogen based plasmas. University of Leoben
Souza Filho IR, Ma Y, Kulse M, Ponge D, Gault B, Springer H et al (2021) Sustainable steel through hydrogen plasma reduction of iron ore: process, kinetics, microstructure, chemistry. Acta Mater 213:116971
Geerdes M, Chaigneau R, Lingiardi O (2020) Modern blast furnace ironmaking: an introduction. Ios Press, New York
Manuel B (2023) Comparing different syngas for blast furnace ironmaking by using the extended operating line methodology. Fuel 333(1):1265331–1265413
Sfi R, Hauke S, Yan M, Ankita M et al (2022) Green steel at its crossroads: Hybrid hydrogen-based reduction of iron ores. J Clean Prod 340(15):1308051–1308115
Jozwiak W, Kaczmarek E, Maniecki T, Ignaczak W, Maniukiewicz W (2007) Reduction behavior of iron oxides in hydrogen and carbon monoxide atmospheres. Appl Catal A 326(1):17–27
Yan M, R. SFI, Xue Z, Supriya N, Pere BV, Guillermo R, et al (2022) Hydrogen-based direct reduction of iron oxide at 700°C: heterogeneity at pellet and microstructure scales. Int J Miner Metall Mater 29(10):1901–1907
Yan M et al (2022) Hierarchical nature of hydrogen-based direct reduction of iron oxides. Scr Mater 1:114571
Zhenxiao X, Zeyi J, Xinru Z, Zhen L, Yuanxiang L, Yewei H et al (2022) The CO2 reduction potential for the oxygen blast furnace with CO2 capture and storage under hydrogen-enriched conditions. Int J Gas Control 121:1
Ea A, Sl T, SeHo K, Baptiste G, Dierk R (2023) The fate of water in hydrogen-based iron oxide reduction. Adv Sci 10(24):e2300626
Spreitzer D, Schenk J (2019) Reduction of iron oxides with hydrogen—a review. Steel Res Int 90(10):1900108
Wei Z, Jing D, Chengzhi L, Xiaobing Y, Zhengliang X, Henrik S (2020) A review on explorations of the oxygen blast furnace process. Steel Res Int 92:1
Ubando AT, Chen WH, Show PL, Ong HC (2020) Kinetic and thermodynamic analysis of iron oxide reduction by graphite for CO2 mitigation in chemical-looping combustion. Int J Energy Res 44(5):3865–3882
Mondal K, Lorethova H, Hippo E, Wiltowski T, Lalvani S (2004) Reduction of iron oxide in carbon monoxide atmosphere—reaction controlled kinetics. Fuel Process Technol 86(1):33–47
Barde AA, Klausner JF, Mei R (2016) Solid state reaction kinetics of iron oxide reduction using hydrogen as a reducing agent. Int J Hydrogen Energy 41(24):10103–10119
Zhang X (2022) Kinetic analysis of iron ore powder reaction with hydrogen-carbon monoxide. J Miner Metall Mater 29:1882–1890
Zuo H-b, Wang C, Dong J-j, Jiao K-x, Xu R-s (2015) Reduction kinetics of iron oxide pellets with H2 and CO mixtures. Int J Miner Metall Mater 22(7):688–696
Aidin H, Niusha N, Mikko I, Timo F (2021) A review on the kinetics of iron ore reduction by hydrogen. Materials 14(24):7540–7540
Li ZZTCWZW (2022) Direct reduction swelling behavior of pellets in hydrogen-based shaft furnaces under typical atmospheres. J Miner Metall Mater 29:1891–1900
Metolina P, Ribeiro TR, Guardani R (2022) Hydrogen-based direct reduction of industrial iron ore pellets: statistically designed experiments and computational simulation. Int J Miner Metall Mater 29:1908–1921
Thomas W, Daniel S, Johannes S (2022) Using iron ore ultra-fines for hydrogen-based fluidized bed direct reduction—a mathematical evaluation. Materials 15:1
Pineau A, Kanari N, Gaballah I (2006) Kinetics of reduction of iron oxides by H2: part I: low temperature reduction of hematite. Thermochim Acta 447(1):89–100
Pineau A, Kanari N, Gaballah I (2007) Kinetics of reduction of iron oxides by H2: Part II. Low temperature reduction of magnetite. Thermochim Acta 456(2):75–88
Ueno H, Endo S, Tomomura S, Ishiwata N (2015) Outline of CO2 ultimate reduction in steelmaking process by innovative technology for cool earth 50 (COURSE50 project). J Jpn Inst Energy 94:1277–1283
Tonomura S (2013) Outline of course 50. Energy Proc 37:7160–7167
Quader MA, Ahmed S, Ghazilla RAR, Ahmed S, Dahari M (2015) A comprehensive review on energy efficient CO2 breakthrough technologies for sustainable green iron and steel manufacturing. Renew Sustain Energy Rev 50:594–614
Zhang N, Zhou P, Choi Y (2013) Energy efficiency, CO2 emission performance and technology gaps in fossil fuel electricity generation in Korea: a meta-frontier non-radial directional distance functionanalysis. Energy Policy 56:653–662
Choi J, Cho H, Yun S, Jang M-G, Oh S-Y, Binns M et al (2019) Process design and optimization of MEA-based CO2 capture processes for non-power industries. Energy 185:971–980
Jeong S-J (2015) System dynamics approach for the impacts of FINEX technology and carbon taxes on steel demand: case study of the POSCO. Int J Precis Eng Manuf Green Technol 2:85–93
Ariyama T, Takahashi K, Kawashiri Y, Nouchi T (2019) Diversification of the ironmaking process toward the long-term global goal for carbon dioxide mitigation. J Sustain Metall 5(3):276–294
Tang J, Chu M-s, Li F, Feng C, Liu Z-g, Zhou Y-s (2020) Development and progress on hydrogen metallurgy. Int J Miner Metall Mater 27(6):713–723
Bhaskar A, Assadi M, Nikpey SH (2020) Decarbonization of the iron and steel industry with direct reduction of iron ore with green hydrogen. Energies 13(3):758
Ren L, Zhou S, Peng T, Ou X (2021) A review of CO2 emissions reduction technologies and low-carbon development in the iron and steel industry focusing on China. Renew Sustain Energy Rev 143:110846
Lin Y, Yang H, Ma L, Li Z, Ni W (2021) Low-carbon development for the iron and steel industry in China and the world: status quo, future vision, and key actions. Sustainability 13:12548
Zhao J, Zuo H, Wang Y, Wang J, Xue Q (2020) Review of green and low-carbon ironmaking technology. Ironmaking Steelmaking 47(3):296–306
Pei M, Petäjäniemi M, Regnell A, Wijk O (2020) Toward a fossil free future with HYBRIT: development of iron and steelmaking technology in Sweden and Finland. Metals 10(7):972
Varling AS, Christensen TH, Bisinella V (2023) Life cycle assessment of alternative biogas utilisations, including carbon capture and storage or utilisation. Waste Manag 157:168
ArcelorMittal (2019) World first for steel: ArcelorMittal investigates the industrial use of pure hydrogen-ArcelorMittal. https://corporate.arcelormittal.com/news-and-media/news/2019/mar/28-03-2019
Langner AL, L (2019) ArcelorMittal (2023) The carbon reduction potential of hydrogen in the low carbon transition of the iron and steel industry: the case of China. Renewable and Sustainable Energy Reviews 171 in Hamburg. https://corporate.arcelormittal.com/news-andmedia/news/2019/sep/16-09-2019
Vaughan A (2019) Zero carbon’s hard problem. New Sci 244(3256):38–41
Kar SK, Sinha ASK, Harichandan S, Bansal R, Balathanigaimani MS (2022) Hydrogen economy in India: a status review. Wiley Interdiscipl Rev 1:e459
Buergler T, Prammer J (2019) Hydrogen steelmaking: technology options and R&D projects. BHM 164(11):447–451
Wang Y, Zuo H, Zhao J (2020) Recent progress and development of ironmaking in China as of 2019: an overview. Ironmaking Steelmaking 47(6):640–649
Li J, Kuang S, Jiao L, Liu L, Zou R, Yu A (2022) Numerical modeling and analysis of hydrogen blast furnace ironmaking process. Fuel 323:1243681–1243716
Nishioka K, Ujisawa Y, Tonomura S, Ishiwata N, Sikstrom P (2016) Sustainable aspects of CO2 ultimate reduction in the steelmaking process (COURSE50 project), part 1: hydrogen reduction in the blast furnace. J Sustain Metall 2(3):200–208
Onoda M, Matsuzaki Y, Chowdhury FA, Yamada H, Goto K, Tonomura S (2016) Sustainable aspects of ultimate reduction of CO2 in the steelmaking process (COURSE50 project), part 2: CO2 capture. J Sustain Metall 2(3):209–215
Junjie Y (2018) Progress and future of breakthrough low-carbon steelmaking technology (ULCOS) of EU. Int J Miner Process Extract Metall 3(2):15–15
Quader MA, Ahmed S, Dawal SZ, Nukman Y (2016) Present needs, recent progress and future trends of energy-efficient ultra-low carbon dioxide (CO2) steelmaking (ULCOS) program. Renew Sustain Energy Rev 55:537–549
Alexandra H, Jan VDS, Dominique S (2012) ULCOS top gas recycling blast furnace. Stahl Undsen 132(4):31–40
World Steel Capacity News (2023) EU commission approves funds for Thyssenkrupp's tkH2Steel Programme. World Steel Capacity News
Peng S, Binglang R, Jingsong W, Guang W, Haibin Z, Qingguo X (2023) Current situation and development prospects of metallurgical by-product gas utilization in China’s steel industry. Int J Hydrogen Energy 48(74):28945–28969
Yichao H, Yinxuan Q, Jian C, Liangyuan H, Edward RT, Victor R et al (2022) Integrating a top-gas recycling and CO2 electrolysis process for H2-rich gas injection and reduce CO2 emissions from an ironmaking blast furnace. Materials 15(6):2008–2008
Michael B, Kyriakos P, Panos S, Spyridon V, Ismael M, Alice P et al (2021) Integration of renewable hydrogen production in steelworks off-gases for the synthesis of methanol and methane. Energies 14(10):2904–2904
Joakim A, Stefan G (2021) A comparison of two hydrogen storages in a fossil-free direct reduced iron process. Int J Hydrogen Energy 46(56):28657–28674
Author information
Authors and Affiliations
Contributions
MS is the corresponding author contributed toward writing—review and editing. KP contributed toward review and editing. MB contributed toward conceptualization and supervision. XM contributed toward review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
The contributing editor for this article was Yan Ma.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, M., Pang, K., Barati, M. et al. Hydrogen-Based Reduction Technologies in Low-Carbon Sustainable Ironmaking and Steelmaking: A Review. J. Sustain. Metall. 10, 10–25 (2024). https://doi.org/10.1007/s40831-023-00772-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00772-4