Abstract
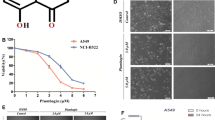
Selenium-containing agents showed novel anticancer activity by triggering pro-oxidative mechanism. Studies confirmed that methylseleninic acid (MeSe) displayed broad-spectrum anti-tumor activity against kinds of human cancers. However, the anticancer effects and mechanism of MeSe against human glioma growth have not been explored yet. Herein, the present study showed that MeSeA dose-dependently inhibited U251 and U87 human glioma cells growth in vitro. Flow cytometry analysis indicated that MeSe induced significant U251 cells apoptosis with a dose-dependent manner, followed by the activation of caspase-7, caspase-9 and caspase-3. Immunofluorescence staining revealed that MeSe time-dependently caused reactive oxide species (ROS) accumulation and subsequently resulted in oxidative damage, as convinced by the increased phosphorylation level of Ser428-ATR, Ser1981-ATM, Ser15-p53 and Ser139-histone. ROS inhibition by glutathione (GSH) effectively attenuated MeSe-induced ROS generation, oxidative damage, caspase-3 activation and cytotoxicity, indicating that ROS was an upstream factor involved in MeSe-mediated anticancer mechanism in glioma. Importantly, MeSe administration in nude mice significantly inhibited glioma growth in vivo by inducing apoptosis through triggering oxidative damage. Taken together, our findings validated the possibility that MeSe as a selenium-containing can act as potential tumor chemotherapy agent for therapy of human glioma.





Similar content being viewed by others
Data availability
All data used to support the findings of this study are included within the article.
Code availability
Not applicable.
References
Anderson SL, Townsend HGG, Singh B (2018) Role of toll-like receptor 4 and caspase-3, -8, and – 9 in lipopolysaccharide-induced delay of apoptosis in equine neutrophils. Am J Vet Res 79:424–432. https://doi.org/10.2460/ajvr.79.4.424
Behera C, Sandha KK, Banjare N, Malik SB, Tabassum M, Kumar R, Kumar A, Mondhe DM, Gupta PN (2022) Implication of methylselenocysteine in combination chemotherapy with gemcitabine for improved anticancer efficacy. Eur J Pharm Sciences: Official J Eur Federation Pharm Sci 176:106238. https://doi.org/10.1016/j.ejps.2022.106238
Brenneisen P, Steinbrenner H, Sies H (2005) Selenium, oxidative stress, and health aspects. Mol Aspects Med 26:256–267. https://doi.org/10.1016/j.mam.2005.07.004
Diogo CV, Suski JM, Lebiedzinska M, Karkucinska-Wieckowska A, Wojtala A, Pronicki M, Duszynski J, Pinton P, Portincasa P, Oliveira PJ, Wieckowski MR (2013) Cardiac mitochondrial dysfunction during hyperglycemia–the role of oxidative stress and p66Shc signaling. Int J Biochem Cell Biol 45:114–122. https://doi.org/10.1016/j.biocel.2012.07.004
Hu W, Ma Y, Zhao C, Yin S, Hu H (2021) Methylseleninic acid overcomes programmed death-ligand 1-mediated resistance of prostate cancer and lung cancer. Mol Carcinog 60:746–757. https://doi.org/10.1002/mc.23340
Ip C, Thompson HJ, Zhu Z, Ganther HE (2000) In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res 60:2882–2886
Jiang T, Nam DH, Ram Z, Poon WS, Wang J, Boldbaatar D, Mao Y, Ma W, Mao Q, You Y, Jiang C, Yang X, Kang C, Qiu X, Li W, Li S, Chen L, Li X, Liu Z, Wang W, Bai H, Yao Y, Li S, Wu A, Sai K, Li G, Yao K, Wei X, Liu X, Zhang Z, Dai Y, Lv S, Wang L, Lin Z, Dong J, Xu G, Ma X, Zhang W, Zhang C, Chen B, You G, Wang Y, Wang Y, Bao Z, Yang P, Fan X, Liu X, Zhao Z, Wang Z, Li Y, Wang Z, Li G, Fang S, Li L, Liu Y, Liu S, Shan X, Liu Y, Chai R, Hu H, Chen J, Yan W, Cai J, Wang H, Chen L, Yang Y, Wang Y, Han L, Wang Q Chinese Glioma Cooperative G, Society for Neuro-Oncology of C, Chinese Brain Cancer A, Chinese Glioma Genome A, Asian Glioma Genome Atlas n (2021) clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett 499:60–72. https://doi.org/10.1016/j.canlet.2020.10.050
Lipinski B (2019) Redox-active selenium in Health and Disease: a conceptual review. Mini Rev Med Chem 19:720–726. https://doi.org/10.2174/1389557517666161104125022
Liu CA, Chang CY, Hsueh KW, Su HL, Chiou TW, Lin SZ, Harn HJ (2018) Migration/Invasion of Malignant gliomas and implications for Therapeutic Treatment. Int J Mol Sci 19. https://doi.org/10.3390/ijms19041115
Manea SA, Antonescu ML, Fenyo IM, Raicu M, Simionescu M, Manea A (2018) Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes. Redox Biol 16:332–343. https://doi.org/10.1016/j.redox.2018.03.011
Messaoudi K, Clavreul A, Lagarce F (2015) Toward an effective strategy in glioblastoma treatment. Part I: resistance mechanisms and strategies to overcome resistance of glioblastoma to temozolomide. Drug Discovery Today 20:899–905. https://doi.org/10.1016/j.drudis.2015.02.011
Miller KD, Ostrom QT, Kruchko C, Patil N, Tihan T, Cioffi G, Fuchs HE, Waite KA, Jemal A, Siegel RL, Barnholtz-Sloan JS (2021) Brain and other central nervous system tumor statistics, 2021. Cancer J Clin 71:381–406. https://doi.org/10.3322/caac.21693
Okuno T, Honda E, Arakawa T, Ogino H, Ueno H (2014) Glutathione-dependent cell cycle G1 arrest and apoptosis induction in human lung cancer A549 cells caused by methylseleninic acid: comparison with sodium selenite. Biol Pharm Bull 37:1831–1837. https://doi.org/10.1248/bpb.b14-00453
Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2021) CBTRUS Statistical Report: primary brain and other Central Nervous System tumors diagnosed in the United States in 2014–2018. Neuro Oncol 23:iii1–iii105. https://doi.org/10.1093/neuonc/noab200
Ou A, Yung WKA, Majd N (2020) Molecular mechanisms of Treatment Resistance in Glioblastoma. Int J Mol Sci 22. https://doi.org/10.3390/ijms22010351
Palmeira CM, Rolo AP, Berthiaume J, Bjork JA, Wallace KB (2007) Hyperglycemia decreases mitochondrial function: the regulatory role of mitochondrial biogenesis. Toxicol Appl Pharmcol 225:214–220. https://doi.org/10.1016/j.taap.2007.07.015
Qiu C, Zhang T, Zhu X, Qiu J, Jiang K, Zhao G, Wu H, Deng G (2019) Methylseleninic acid suppresses breast Cancer Growth via the JAK2/STAT3 pathway. Reproductive Sci 26:829–838. https://doi.org/10.1177/1933719118815582
Rayman MP (2012) Selenium and human health. Lancet 379:1256–1268. https://doi.org/10.1016/S0140-6736(11)61452-9
Rolo AP, Palmeira CM (2006) Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmcol 212:167–178. https://doi.org/10.1016/j.taap.2006.01.003
Sampson JH, Gunn MD, Fecci PE, Ashley DM (2020) Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer 20:12–25. https://doi.org/10.1038/s41568-019-0224-7
Spallholz JE, Palace VP, Reid TW (2004) Methioninase and selenomethionine but not Se-methylselenocysteine generate methylselenol and superoxide in an in vitro chemiluminescent assay: implications for the nutritional carcinostatic activity of selenoamino acids. Biochem Pharmacol 67:547–554. https://doi.org/10.1016/j.bcp.2003.09.004
Tarrado-Castellarnau M, Cortes R, Zanuy M, Tarrago-Celada J, Polat IH, Hill R, Fan TW, Link W, Cascante M (2015) Methylseleninic acid promotes antitumour effects via nuclear FOXO3a translocation through akt inhibition. Pharmacol Res 102:218–234. https://doi.org/10.1016/j.phrs.2015.09.009
Varlamova EG, Turovsky EA (2021) The Main Cytotoxic effects of Methylseleninic Acid on various Cancer cells. Int J Mol Sci 22. https://doi.org/10.3390/ijms22126614
Wang L, Hu H, Wang Z, Xiong H, Cheng Y, Liao JD, Deng Y, Lu J (2014) Methylseleninic acid suppresses pancreatic cancer growth involving multiple pathways. Nutr Cancer 66:295–307. https://doi.org/10.1080/01635581.2014.868911
Wang XJ, Chen W, Fu XT, Ma JK, Wang MH, Hou YJ, Tian DC, Fu XY, Fan CD (2019) Reversal of homocysteine-induced neurotoxicity in rat hippocampal neurons by astaxanthin: evidences for mitochondrial dysfunction and signaling crosstalk. Cell Death Discover 4:50. https://doi.org/10.1038/s41420-018-0114-x
Yang K, Wu Z, Zhang H, Zhang N, Wu W, Wang Z, Dai Z, Zhang X, Zhang L, Peng Y, Ye W, Zeng W, Liu Z, Cheng Q (2022) Glioma targeted therapy: insight into future of molecular approaches. Mol Cancer 21:39. https://doi.org/10.1186/s12943-022-01513-z
Zhu Z, Du S, Du Y, Ren J, Ying G, Yan Z (2018) Glutathione reductase mediates drug resistance in glioblastoma cells by regulating redox homeostasis. J Neurochem 144:93–104. https://doi.org/10.1111/jnc.14250
Funding
This study was supported by Medical Health Science and Technology Project of Shandong Province (202302021265), Key Research and Development Project of Linyi City (2023YX0008) and Natural Science Foundation of Shandong (ZR2022MH106).
Author information
Authors and Affiliations
Contributions
XJ, XY and JL designed the study. WC. XT, QL and PD prepared and revised the manuscript. QL, XZ, JC and ZX contributed to the quality assessment, data analysis, and interpretation of the data. All authors revised the manuscript and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval
The animal experiments were approved and conducted by the Animal Care and Use Committee of Second Affiliated Hospital of Shandong First Medical University.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, W., Hao, P., Song, Q. et al. Methylseleninic acid inhibits human glioma growth in vitro and in vivo by triggering ROS-dependent oxidative damage and apoptosis. Metab Brain Dis 39, 625–633 (2024). https://doi.org/10.1007/s11011-024-01344-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-024-01344-5