Abstract
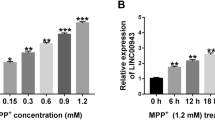
Parkinson’s disease (PD) is an age-related neurodegenerative disease. Long non-coding RNA urothelial carcinoma-associated 1 (UCA1) is involved in the pathogenesis of PD. However, the pathogenesis of PD regulated by UCA1 has not been fully explained. We used 1-Methyl-4-phenylpyridinium (MPP+)-induced SK-N-SH cells for functional analysis. Expression levels of UCA1, microRNA (miR)-671-5p, and KPNA4 (karyopherin subunit alpha 4) mRNA were detected using quantitative real-time polymerase chain reaction (qRT-PCR). Cell viability and apoptosis were analyzed using MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) or flow cytometry assays. Some protein levels were measured by western blotting. The levels of pro-inflammatory cytokines were tested by ELISA (enzyme-linked immunosorbent assay). The levels of LDH (lactate dehydrogenase), MDA (malondialdehyde), and SOD (superoxide dismutase) were measured using corresponding kits. The relationship between UCA1 or KPNA4 and miR-671-5p was verified by dual-luciferase reporter assay and/or RNA immunoprecipitation (RIP) assay. MPP+ induced UCA1 expression in SK-N-SH cells in a concentration-dependent manner or time-dependent manner. UCA1 knockdown reduced MPP+-induced apoptosis, inflammation, and oxidative stress in SK-N-SH cells. MiR-671-5p was downregulated while KPNA4 was upregulated in MPP+-treated SK-N-SH cells. UCA1 sponged miR-671-5p to regulate KPNA4 expression. MiR-671-5p inhibition counteracted UCA1 knockdown-mediated influence on apoptosis, inflammation, and oxidative stress of MPP+-induced SK-N-SH cells. KPNA4 overexpression offset the inhibitory influence of miR-671-5p mimic on apoptosis, inflammation, and oxidative stress of MPP+-treated SK-N-SH cells. UCA1 inhibition reduced MPP+-induced neuronal damage through the miR-671-5p/KPNA4 pathway in SK-N-SH cells, providing a novel mechanism to understand the pathogenesis of PD.






Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Cacabelos R (2017) Parkinson’s, Disease: From pathogenesis to pharmacogenomics. Int J Mol Sci 18(3):551
Cai L, Tu L, Li T, Yang X, Ren Y, Gu R, Zhang Q, Yao H, Qu X, Wang Q, Tian J (2019) Downregulation of lncRNA UCA1 ameliorates the damage of dopaminergic neurons, reduces oxidative stress and inflammation in Parkinson’s disease through the inhibition of the PI3K/Akt signaling pathway. Int Immunopharmacol 75:105734
Cao Z, Pan X, Yang Y, Huang Y, Shen H-B (2018) The lncLocator: a subcellular localization predictor for long non-coding RNAs based on a stacked ensemble classifier. Bioinf (Oxford England) 34:2185–2194
Chen J, Zeng L, Xia T, Li S, Yan T, Wu S, Qiu G, Liu Z (2015) Toward a biomarker of oxidative stress: a fluorescent probe for exogenous and endogenous malondialdehyde in living cells. Anal Chem 87:8052–8056
Corti O, Lesage S, Brice A (2011) What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol Rev 91:1161–1218
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinsons Dis 3:461–491
Fagerlund R, Kinnunen L, Köhler M, Julkunen I, Melén (2005) K NF-{kappa}B is transported into the nucleus by importin {alpha}3 and importin {alpha}4. J Biol Chem 280:15942–15951
Francelle L, Galvan L, Gaillard M-C, Petit F, Bernay B, Guillermier M, Bonvento G, Dufour N, Elalouf J-M, Hantraye P, Déglon N, de Chaldée M (2015) Brouillet E Striatal long noncoding RNA Abhd11os is neuroprotective against an N-terminal fragment of mutant huntingtin in vivo. Neurobiol Aging 36:1601.e1607-1601.1616
Gao H-M, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VMY (2008) Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci: Off J Soc Neurosci 28:7687–7698
Ghosh A, Tyson T, George S, Hildebrandt EN, Steiner JA, Madaj Z, Schulz E, Machiela E, McDonald WG, Escobar Galvis ML, Kordower JH, Van Raamsdonk JM, Colca JR (2016) Brundin P Mitochondrial pyruvate carrier regulates autophagy, inflammation, and neurodegeneration in experimental models of Parkinson’s disease. Sci Transl Med 8:368ra174
Gonçalves AP, Máximo V, Lima J, Singh KK, Soares P, Videira A (2011) Involvement of p53 in cell death following cell cycle arrest and mitotic catastrophe induced by rotenone. Biochim Biophys Acta 1813:492–499
Hauser DN, Hastings TG (2013) Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol Dis 51:35–42
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397
Hu X, Ma R, Fu W, Zhang C, Du X (2019) LncRNA UCA1 sponges miR-206 to exacerbate oxidative stress and apoptosis induced by ox-LDL in human macrophages. J Cell Physiol 234:14154–14160
Li X, Diao H, Circular (2019) RNA circ_0001946 acts as a competing endogenous RNA to inhibit glioblastoma progression by modulating miR-671-5p and CDR1. J Cell Physiol 234:13807–13819
Lin Q, Hou S, Dai Y, Jiang N, Lin Y (2019) LncRNA HOTAIR targets miR-126-5p to promote the progression of Parkinson’s disease through RAB3IP. Biol Chem 400:1217–1228
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, Calif) 25:402–408
Lu M, Sun WL, Shen J, Wei M, Chen B, Qi YJ, Xu CS (2018) LncRNA-UCA1 promotes PD development by upregulating SNCA. Eur Rev Med Pharmacol Sci 22:7908–7915
Marchenko ND, Hanel W, Li D, Becker K, Reich N, Moll UM (2010) Stress-mediated nuclear stabilization of p53 is regulated by ubiquitination and importin-alpha3 binding. Cell Death Differ 17:255–267
Nakamori M, Junn E, Mochizuki H, Mouradian MM (2019) Nucleic acid-based therapeutics for Parkinson’s disease. Neurotherapeutics 16:287–298
Nan A, Chen L, Zhang N, Liu Z, Yang T, Wang Z, Yang C (2017) Jiang Y A novel regulatory network among LncRpa, CircRar1, MiR-671 and apoptotic genes promotes lead-induced neuronal cell apoptosis. Arch Toxicol 91:1671–1684
Ping X, Cheng Y, Bao J, Shi K, Zou J, Shentu X (2021) KPNA4 is involved in cataract formation via the nuclear import of p53. Gene 786:145621
Rashid F, Shah A, Shan G (2016) Long non-coding RNAs in the cytoplasm. Genom Proteom Bioinform 14:73–80
Salmena L, Poliseno L, Tay Y, Kats L (2011) Pandolfi PP A ceRNA hypothesis: the Rosetta Stone of a hidden RNA. language? Cell 146:353–358
Savitt JM, Dawson VL, Dawson TM (2006) Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Investig 116:1744–1754
Shi CH, Huang Y, Li WQ, Chen RG (2019) Influence of LncRNA UCA1 on glucose metabolism in rats with diabetic nephropathy through PI3K-Akt signaling pathway. Eur Rev Med Pharmacol Sci 23:10058–10064
Song H, Xu Y, Xu T, Fan R, Jiang T, Cao M, Shi L, Song J (2020) CircPIP5K1A activates KRT80 and PI3K/AKT pathway to promote gastric cancer development through sponging miR-671-5p. J Cell Physiol 126:109941
Subramaniam SR, Chesselet M-F (2013) Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Prog Neurobiol 106–107:17–32
Sun S-C (2017) The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol 17:545–558
Tansey MG, Goldberg MS (2010) Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis 37:510–518
Thomson DW, Dinger ME (2016) Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet 17:272–283
Uwatoko H, Hama Y, Iwata IT, Shirai S, Matsushima M, Yabe I, Utsumi J, Sasaki H (2019) Identification of plasma microRNA expression changes in multiple system atrophy and Parkinson’s disease. Mol Brain 12:49
Wang D-Q, Fu P, Yao C, Zhu L-S, Hou T-Y, Chen J-G, Lu Y, Liu D, Zhu L-Q (2018) Long non-coding RNAs, novel culprits, or bodyguards in neurodegenerative diseases. Mol Ther Nucleic Acids 10:269–276
Wang X-S, Zhang Z, Wang H-C, Cai J-L, Xu Q-W, Li M-Q, Chen Y-C, Qian X-P, Lu T-J, Yu L-Z, Zhang Y, Xin D-Q, Na Y-Q, Chen W-F (2006) Rapid identification of UCA1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res 12:4851–4858
Xie X-X, Kou S-T, Pu Z-H, Hou C-Y, Tian Y-P (2007) Effects of scalp catgut embedding on SOD, NO, MDA in the rat with Parkinson’s disease. Zhongguo Zhen Jiu = Chin Acupuncture & Moxibustion 27:753–756
Xing R-X, Li L-G, Liu X-W, Tian B-X, Cheng Y (2020) Down regulation of miR-218, miR-124, and miR-144 relates to Parkinson’s disease via activating NF-κB signaling. Kaohsiung J Med Sci 36:786–792
Xu Z, Cawthon D, McCastlain KA, Duhart HM, Newport GD, Fang H, Patterson TA, Slikker W Jr (2005) Ali SF Selective alterations of transcription factors in MPP+-induced neurotoxicity in PC12 cells. Neurotoxicology 26:729–737
Xue M, Chen W, Li X (2016) Urothelial cancer associated 1: a long noncoding RNA with a crucial role in cancer. J Cancer Res Clin Oncol 142:1407–1419
Yi J, Chen B, Yao X, Lei Y, Ou F, Huang F (2019) Upregulation of the lncRNA MEG3 improves cognitive impairment, alleviates neuronal damage, and inhibits activation of astrocytes in hippocampus tissues in Alzheimer’s disease through inactivating the PI3K/Akt signaling pathway. J Cell Biochem 120:18053–18065
Zella MAS, Metzdorf J, Ostendorf F, Maass F, Muhlack S, Gold R, Haghikia A, Tönges L (2019) Novel Immunotherapeutic Approaches to Target Alpha-Synuclein and Related Neuroinflammation in Parkinson’s disease. Cells 8(2):105
Author information
Authors and Affiliations
Contributions
Zhengheng Hao designed and performed the research; Wen Dang, Qingfeng Zhu, Jianxing Xu analyzed the data; Zhengheng Hao wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consents were obtained from all participants and this study was permitted by the Ethics Committee of the Second Hospital of Shanxi Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

Supplementary Fig. 1
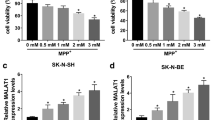
(A) Protein levels of p-p65 and p65 in SK-N-SH cells at different groups (Control, MMP+, MMP++miR-NC, MMP++miR-671-5p, MMP++miR-671-5p + vector, or MMP++miR-671-5p + KPNA4) were detected. (C) The viability of SK-N-SH cells treated with different concentrations of MPP+ (0, 0.5, 1, 2, 4 mM) was determined. (C) Transfection efficiencies of three siRNAs were evaluated. *P < 0.05. (PNG 320 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hao, Z., Dang, W., Zhu, Q. et al. Long non-coding RNA UCA1 regulates MPP+-induced neuronal damage through the miR-671-5p/KPNA4 pathway in SK-N-SH cells. Metab Brain Dis 38, 961–972 (2023). https://doi.org/10.1007/s11011-022-01118-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01118-x