Abstract
Background
Parkinson’s disease (PD) is a neurodegenerative disease resulted from the loss of dopaminergic neurons. Here, we analyzed the role of long noncoding RNA (lncRNA) small nucleolar RNA host gene 14 (SNHG14) in PD using 1-methyl-4-phenyl pyridine (MPP+)-induced PD cell model.
Methods
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and Western blot assay were performed to determine RNA and protein expression, respectively. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and flow cytometry (FCM) analysis were conducted to analyze cell viability and apoptosis. Enzyme-Linked Immunosorbent Assay (ELISA) was conducted to analyze the release of inflammatory cytokines. Cytotoxicity was assessed using reactive oxygen species (ROS) assay kit, superoxide dismutase (SOD) activity assay kit and lactate dehydrogenase (LDH) activity assay kit. Dual-luciferase reporter assay and RNA immunoprecipitation (RIP) assay were performed to confirm the interaction between microRNA-135b-5p (miR-135b-5p) and SNHG14 or karyopherin subunit alpha 4 (KPNA4).
Results
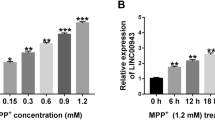
MPP+ treatment elevated the expression of SNHG14 in SK-N-SH cells in a dose and time-dependent manner. SNHG14 knockdown alleviated MPP+-induced apoptosis, inflammation, and cytotoxicity in SK-N-SH cells. SNHG14 interacted with miR-135b-5p, and SNHG14 silencing-mediated effects were partly overturned by miR-135b-5p knockdown in PD cell model. Besides, miR-135b-5p interacted with the 3’ untranslated region (3’UTR) of KPNA4, and KPNA4 overexpression partly reversed miR-135b-5p overexpression-induced effects in PD cell model. SNHG14 knockdown reduced the protein level of KPNA4 partly by up-regulating miR-135b-5p in SK-N-SH cells.
Conclusion
SNHG14 promoted MPP+-induced neuro injury in PD cell model through mediating miR-135b-5p/KPNA4 axis.








Similar content being viewed by others
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Andican G, Konukoglu D, Bozluolcay M, Bayülkem K, Firtiına S, Burcak G (2012) Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol Belg 112(2):155–159
Binukumar BK, Shukla V, Amin ND, Grant P, Bhaskar M, Skuntz S et al (2015) Peptide TFP5/TP5 derived from Cdk5 activator P35 provides neuroprotection in the MPTP model of Parkinson’s disease. Mol Biol Cell 26(24):4478–4491
Cai LJ, Tu L, Huang XM, Huang J, Qiu N, Xie GH et al (2020) LncRNA MALAT1 facilitates inflammasome activation via epigenetic suppression of Nrf2 in Parkinson’s disease. Mol Brain 13(1):130
Carvey PM, Punati A, Newman MB (2006) Progressive dopamine neuron loss in Parkinson’s disease: the multiple hit hypothesis. Cell Transplant 15(3):239–250
de Lau LM, Breteler MM (2006) Epidemiology of Parkinson’s disease. Lancet Neurol 5(6):525–535
Feng L, Wang R, Yang Y, Shen X, Shi Q, Lian M et al (2021) KPNA4 regulated by miR-548b-3p promotes the malignant phenotypes of papillary thyroid cancer. Life Sci 265:118743
Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8(4):382–397
Hu RH, Zhang ZT, Wei HX, Ning L, Ai JS, Li WH et al (2020) LncRNA ST7-AS1, by regulating miR-181b-5p/KPNA4 axis, promotes the malignancy of lung adenocarcinoma. Cancer Cell Int 20(1):568
Jiang X, Jin T, Zhang H, Miao J, Zhao X, Su Y et al (2019) Current Progress of Mitochondrial Quality Control Pathways Underlying the Pathogenesis of Parkinson’s Disease. Oxid Med Cell Longev 2019:4578462
Jiang H, Ni J, Zheng Y, Xu Y (2021) Knockdown of lncRNA SNHG14 alleviates LPS-induced inflammation and apoptosis of PC12 cells by regulating miR-181b-5p. Exp Ther Med 21(5):497
Li X, Liu J, Liu M, Xia C, Zhao Q (2020) The Lnc LINC00461/miR-30a-5p facilitates progression and malignancy in non-small cell lung cancer via regulating ZEB2. Cell Cycle 19(7):825–836
Li X, Su Y, Li N, Zhang FR, Zhang N (2021) Berberine Attenuates MPP(+)-Induced Neuronal Injury by Regulating LINC00943/miR-142-5p/KPNA4/NF-kappaB Pathway in SK-N-SH Cells. Neurochem Res 46(12):3286–3300
Liu XH, Sun M, Nie FQ, Ge YB, Zhang EB, Yin DD et al (2014) Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer 13:92
Muniategui A, Nogales-Cadenas R, Vázquez M, Aranguren XL, Agirre X, Luttun A et al (2012) Quantification of miRNA-mRNA interactions. PLoS ONE 7(2):e30766
Paraskevopoulou MD, Hatzigeorgiou AG (2016) Analyzing MiRNA-LncRNA Interactions. Methods Mol Biol 1402:271–286
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J et al (2017) Parkinson Disease Nat Rev Dis Primers 3:17013
Veneziano D, Marceca GP, Di Bella S, Nigita G, Distefano R, Croce CM (2019) Investigating miRNA-lncRNA Interactions: Computational Tools and Resources. Methods Mol Biol 1970:251–277
Whitton PS (2007) Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br J Pharmacol 150(8):963–976
Wilusz JE, Sunwoo H, Spector DL (2009) Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 23(13):1494–1504
Xing RX, Li LG, Liu XW, Tian BX, Cheng Y (2020) Down regulation of miR-218, miR-124, and miR-144 relates to Parkinson’s disease via activating NF-κB signaling. Kaohsiung J Med Sci 36(10):786–792
Yang J, Lu C, Wei J, Guo Y, Liu W, Luo L et al (2017) Inhibition of KPNA4 attenuates prostate cancer metastasis. Oncogene 36(20):2868–2878
Zhang J, Liu W, Wang Y, Zhao S, Chang N (2017) miR-135b Plays a Neuroprotective Role by Targeting GSK3β in MPP(+)-Intoxicated SH-SY5Y Cells. Dis Markers 2017:5806146
Zhang LM, Wang MH, Yang HC, Tian T, Sun GF, Ji YF et al (2019a) Dopaminergic neuron injury in Parkinson’s disease is mitigated by interfering lncRNA SNHG14 expression to regulate the miR-133b/ α-synuclein pathway. Aging (albany NY) 11(21):9264–9279
Zhang M, Luo H, Hui L (2019b) MiR-3619-5p hampers proliferation and cisplatin resistance in cutaneous squamous-cell carcinoma via KPNA4. Biochem Biophys Res Commun 513(2):419–425
Zhang Y, Xia Q, Lin J (2020) LncRNA H19 Attenuates Apoptosis in MPTP-Induced Parkinson’s Disease Through Regulating miR-585-3p/PIK3R3. Neurochem Res 45(7):1700–1710
Zhao CC, Jiao Y, Zhang YY, Ning J, Zhang YR, Xu J et al (2019) Lnc SMAD5-AS1 as ceRNA inhibit proliferation of diffuse large B cell lymphoma via Wnt/β-catenin pathway by sponging miR-135b-5p to elevate expression of APC. Cell Death Dis 10(4):252
Zhou S, Zhang D, Guo J, Zhang J, Chen Y (2020) Knockdown of SNHG14 Alleviates MPP(+)-Induced Injury in the Cell Model of Parkinson’s Disease by Targeting the miR-214-3p/KLF4 Axis. Front Neurosci 14:930
Acknowledgements
None.
Funding
There is no funding to report.
Author information
Authors and Affiliations
Contributions
JF designed and supervised the study. XY was a major contributor in writing the manuscript. YW collected and analyzed the data. LL contributed the methodology and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Supplementary Figure 1. Agarose gel electrophoresis was used to confirm the specificity of KPNA4 (one band, 138bp), U6 (one band, 94bp) and GAPDH (one band, 258bp) primers. Supplementary Figure 2. SNHG14 knockdown alleviates MPP+-induced injury in SK-N-SH cells. (A) RT-qPCR was conducted to evaluate the transfection efficiency of LNA-GapmeR in SK-N-SH cells (N=3). (B) Cell viability was analyzed using MTT assay (N=3). (C) FCM analysis was used to measure cell apoptosis rate (N=3). (D and E) ELISA was used to detect the levels of TNF-α and IL-6 in the culture supernatant. (F-H) The generation of ROS and the activities of SOD and LDH were measured using their corresponding kits. A, Student’s t-test; B-H, one-way ANOVA. *P<0.05.
Rights and permissions
About this article
Cite this article
Yuan, X., Wu, Y., Lu, L. et al. Long noncoding RNA SNHG14 knockdown exerts a neuroprotective role in MPP+-induced Parkinson’s disease cell model through mediating miR-135b-5p/KPNA4 axis. Metab Brain Dis 37, 2363–2373 (2022). https://doi.org/10.1007/s11011-022-01038-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01038-w