Abstract
This study aimed to investigate the protective effects of the alpha-2 adrenergic receptor (α2-AR) agonist, clonidine, on the cerebral ischemia–reperfusion (I/R) injury and elaborate the underlying mechanisms. Cerebral I/R model was established by middle cerebral artery occlusion (MCAO) for 2 h followed by reperfusion for 4 h in adult male SD rats. Saline, clonidine and yohimbine (an α2-AR antagonist) were intraperitoneally administered each day for one week before surgery. Neurological deficit was evaluated just before decapitation. TTC staining was applied for correlation of cerebral infarction volume. HE staining was performed to observe the neuron morphology. Immunohistochemical staining was performed to detect the localization and expression of GluN3 proteins. Western blot analysis also was used to detect the expression levels of GluN3 proteins. Our data showed that clonidine ameliorated neurological deficit and reduced the cerebral infarction volume of the rats with cerebral I/R. It is worth noting that treatment with clonidine up-regulated the protein expression of GluN3 in the rats with the cerebral I/R, especially in the cell membrane. Moreover, clonidine also up-regulated the transposition from cytoplasm to cell membrane of GluN3 after cerebral I/R. In addition, yohimbine abolished the neuroprotective effects of clonidine. The results indicated that clonidine played a protective role in cerebral I/R injury through regulation of the protein expression of GluN3 subunits of N-methyl-D-aspartate (NMDA) receptor.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- I/R:
-
Ischemia–reperfusion
- α2-AR:
-
Alpha-2 adrenergic receptor
- NMDA:
-
N-methyl-D-aspartate
- Glu:
-
Glutamate
- TTC:
-
2,3,5-Triphenyltetrazolium chloride
- HE:
-
Hematoxylin-Eosin
- BSA:
-
Bovine serum albumin
- PBS:
-
Phosphate-buffered saline
- DAB:
-
3,3'-Diaminobenzidine
- IOD:
-
Integral optical density
- tPA:
-
Issue-type plasminogen activator
References
Ahad MA, Kumaran KR, Ning T, Mansor NI, Effendy MA, Damodaran T, Lingam K, Wahab HA, Nordin N, Liao P, Müller CP, Hassan Z (2020) Insights into the neuropathology of cerebral ischemia and its mechanisms. Rev Neurosci 31(5):521–538. https://doi.org/10.1515/revneuro-2019-0099
Andersson O, Stenqvist A, Attersand A, von Euler G (2001) Nucleotide sequence, genomic organization, and chromosomal localization of genes encoding the human NMDA receptor subunits NR3A and NR3B. Genomics 78(3):178–184. https://doi.org/10.1006/geno.2001.6666
Bendel O, Prunell G, Stenqvist A, Mathiesen T, Holmin S, Svendgaard NA, Gv E (2005) Experimental subarachnoid hemorrhage induces changes in the levels of hippocampal NMDA receptor subunit mRNA. Brain Res Mol Brain Res 137(1–2):119–125. https://doi.org/10.1016/j.molbrainres.2005.02.023
Cheng NT, Kim AS (2015) Intravenous thrombolysis for acute ischemic stroke within 3 hours versus between 3 and 4.5 hours of symptom onset. Neurohospitalist. 5(3):101–9. https://doi.org/10.1177/1941874415583116
Cheng X, Yang YL, Li WH, Liu M, Zhang SS, Wang YH, Du GH (2020) Dynamic alterations of brain injury, functional recovery, and metabolites profile after cerebral ischemia/reperfusion in rats contributes to potential biomarkers. J Mol Neurosci 70(5):667–676. https://doi.org/10.1007/s12031-019-01474-x
Chung C, Marson JD, Zhang QG, Kim J, Wu WH, Brann DW, Chen BS (2016) Neuroprotection mediated through GluN2C-Containing N-methyl-D-aspartate (NMDA) receptors following ischemia. Sci Rep 6:37033. https://doi.org/10.1038/srep37033
Dean JM, George S, Naylor AS, Mallard C, Gunn AJ, Bennet L (2008) Partial neuroprotection with low-dose infusion of the alpha2-adrenergic receptor agonist clonidine after severe hypoxia in preterm fetal sheep. Neuropharmacology 55(2):166–174. https://doi.org/10.1016/j.neuropharm.2008.05.009
Fonnum F (1984) Glutamate: a neurotransmitter in mammalian brain. J Neurochem 42(1):1–11. https://doi.org/10.1111/j.1471-4159.1984.tb09689.x
Fransen PS, Berkhemer OA, Lingsma HF, Beumer D, van den Berg LA, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama À Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van Oostenbrugge RJ, Majoie CB, van der Lugt A, Dippel DW (2016). Multicenter Randomized Clinical Trial of Endovascular Treatment of Acute Ischemic Stroke in the Netherlands Investigators. Time to Reperfusion and Treatment Effect for Acute Ischemic Stroke: A Randomized Clinical Trial. JAMA Neurol 73(2):190–196. https://doi.org/10.1001/jamaneurol.2015.3886. Erratum in: JAMA Neurol
Gall D, Dupont G (2019) Tonic activation of extrasynaptic NMDA receptors decreases intrinsic excitability and promotes bistability in a model of neuronal activity. Int J Mol Sci 21(1):206. https://doi.org/10.3390/ijms21010206
Gardoni F, Di Luca M (2021) Protein-protein interactions at the NMDA receptor complex: From synaptic retention to synaptonuclear protein messengers. Neuropharmacology 190:108551. https://doi.org/10.1016/j.neuropharm.2021.108551
Garthwaite G, Williams GD, Garthwaite J (1992) Glutamate toxicity: An experimental and theoretical analysis. Eur J Neurosci 4(4):353–360. https://doi.org/10.1111/j.1460-9568.1992.tb00882.x
Glasgow NG, Siegler Retchless B, Johnson JW (2015) Molecular bases of NMDA receptor subtype-dependent properties. J Physiol 593(1):83–95. https://doi.org/10.1113/jphysiol.2014.273763
Hansen KB, Yi F, Perszyk RE, Furukawa H, Wollmuth LP, Gibb AJ, Traynelis SF (2018) Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol 150(8):1081–1105. https://doi.org/10.1085/jgp.201812032
Hansen KB, Wollmuth LP, Bowie D, Furukawa H, Menniti FS, Sobolevsky AI, Swanson GT, Swanger SA, Greger IH, Nakagawa T, McBain CJ, Jayaraman V, Low CM, Dell’Acqua ML, Diamond JS, Camp CR, Perszyk RE, Yuan H, Traynelis SF (2021) Structure, function, and pharmacology of glutamate receptor ion channels. Pharmacol Rev 73(4):298–487. https://doi.org/10.1124/pharmrev.120.000131
He Z, Huang L, Wu Y, Wang J, Wang H, Guo L (2008) DDPH: improving cognitive deficits beyond its alpha 1-adrenoceptor antagonism in chronic cerebral hypoperfused rats. Eur J Pharmacol 588(2–3):178–188. https://doi.org/10.1016/j.ejphar.2008.03.060
Henson MA, Roberts AC, Pérez-Otaño I, Philpot BD (2010) Influence of the NR3A subunit on NMDA receptor functions. Prog Neurobiol 91(1):23–37. https://doi.org/10.1016/j.pneurobio.2010.01.004
Jung KI, Kim JH, Park CK (2015) α2-Adrenergic modulation of the glutamate receptor and transporter function in a chronic ocular hypertension model. Eur J Pharmacol 765:274–283. https://doi.org/10.1016/j.ejphar.2015.08.035
Kanazawa M, Takahashi T, Nishizawa M, Shimohata T (2017) Therapeutic Strategies to Attenuate Hemorrhagic Transformation After Tissue Plasminogen Activator Treatment for Acute Ischemic Stroke. J Atheroscler Thromb 24(3):240–253. https://doi.org/10.5551/jat.RV16006
Karakas E, Furukawa H (2014) Crystal structure of a heterotetrameric NMDA receptor ion channel. Science 344(6187):992–997. https://doi.org/10.1126/science.1251915
Lee CH, Lü W, Michel JC, Goehring A, Du J, Song X, Gouaux E (2014) NMDA receptor structures reveal subunit arrangement and pore architecture. Nature 511(7508):191–197. https://doi.org/10.1038/nature13548
Li C, Meng L, Li X, Li D, Jiang LH (2015) Non-NMDAR neuronal Ca(2+)-permeable channels in delayed neuronal death and as potential therapeutic targets for ischemic brain damage. Expert Opin Ther Targets 19(7):879–892. https://doi.org/10.1517/14728222.2015.1021781
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20(1):84–91. https://doi.org/10.1161/01.str.20.1.84
Lopez MS, Vemuganti R (2018) Modeling transient focal ischemic stroke in rodents by intraluminal filament method of middle cerebral artery occlusion. Methods Mol Biol 1717:101–113. https://doi.org/10.1007/978-1-4939-7526-6_9
Matsuda K, Kamiya Y, Matsuda S, Yuzaki M (2002) Cloning and characterization of a novel NMDA receptor subunit NR3B: a dominant subunit that reduces calcium permeability. Brain Res Mol Brain Res 00(1–2):43–52. https://doi.org/10.1016/s0169-328x(02)00173-0
Morris GP, Wright AL, Tan RP, Gladbach A, Ittner LM, Vissel B (2016) A comparative study of variables influencing ischemic injury in the longa and Koizumi methods of intraluminal filament middle cerebral artery occlusion in mice. PLoS ONE 11(2):e0148503. https://doi.org/10.1371/journal.pone.0148503
Nakanishi N, Tu S, Shin Y, Cui J, Kurokawa T, Zhang D, Chen HS, Tong G, Lipton SA (2009) Neuroprotection by the NR3A subunit of the NMDA receptor. J Neurosci 29(16):5260–5265. https://doi.org/10.1523/JNEUROSCI.1067-09.2009
Nishi M, Hinds H, Lu HP, Kawata M, Hayashi Y (2001) Motoneuron-specific expression of NR3B, a novel NMDA-type glutamate receptor subunit that works in a dominant-negative manner. J Neurosci 21(23):RC185. https://doi.org/10.1523/JNEUROSCI.21-23-j0003.2001
Pachernegg S, Strutz-Seebohm N, Hollmann M (2012) GluN3 subunit-containing NMDA receptors: not just one-trick ponies. Trends Neurosci 35(4):240–249. https://doi.org/10.1016/j.tins.2011.11.010
Paoletti P (2011) Molecular basis of NMDA receptor functional diversity. Eur J Neurosci 33(8):1351–1365. https://doi.org/10.1111/j.1460-9568.2011.07628.x
Paschen W (1996) Glutamate excitotoxicity in transient global cerebral ischemia. Acta Neurobiol Exp (Wars) 56(1):313–322
Rothman S (1984) Synaptic release of excitatory amino acid neurotransmitter mediates anoxic neuronal death. J Neurosci 4(7):1884–1891. https://doi.org/10.1523/JNEUROSCI.04-07-01884.1984
Rothman SM, Olney JW (1986) Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann Neurol 19(2):105–111. https://doi.org/10.1002/ana.410190202
Sattler R, Tymianski M (2001) Molecular mechanisms of glutamate receptor-mediated excitotoxic neuronal cell death. Mol Neurobiol 24(1–3):107–129. https://doi.org/10.1385/MN:24:1-3:107
Suzuki H, Kanamaru H, Kawakita F, Asada R, Fujimoto M, Shiba M (2021) Cerebrovascular pathophysiology of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Histol Histopathol 36(2):143–158. https://doi.org/10.14670/HH-18-253
Tameh AA, Karimian M, Zare-Dehghanani Z, Aftabi Y, Beyer C (2018) Role of steroid therapy after ischemic stroke by n-Methyl-d-Aspartate receptor gene regulation. J Stroke Cerebrovasc Dis 27(11):3066–3075. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.06.041
Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R (2010) Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev 62(3):405–496. https://doi.org/10.1124/pr.109.002451.Erratum.In:PharmacolRev
Wang WZ, Yuan WJ, Pan YX, Tang CS, Su DF (2004) Interaction between clonidine and N-methyl-D-aspartate receptors in the caudal ventrolateral medulla of rats. Exp Brain Res 158(2):259–264. https://doi.org/10.1007/s00221-004-1902-5
Wang H, Yan H, Zhang S, Wei X, Zheng J, Li J (2013) The GluN3A subunit exerts a neuroprotective effect in brain ischemia and the hypoxia process. ASN Neuro 5(4):231–242. https://doi.org/10.1042/AN20130009
Yanli L, Xizhou Z, Yan W, Bo Z, Yunhong Z, Zicheng L, Lingling Y, Lingling Y, Zhangao C, Min Z, Zhi H (2016) Clonidine preconditioning alleviated focal cerebral ischemic insult in rats via up-regulating p-NMDAR1 and down-regulating NMDAR2A / p-NMDAR2B. Eur J Pharmacol 793:89–94. https://doi.org/10.1016/j.ejphar.2016.10.036
Zhang X, Shi X, Wang J, Xu Z, He J (2021) Enriched environment remedies cognitive dysfunctions and synaptic plasticity through NMDAR-Ca2+-Activin A circuit in chronic cerebral hypoperfusion rats. Aging (Albany NY) 13(16):20748–20761. https://doi.org/10.18632/aging.203462
Zhao H, Steinberg GK, Sapolsky RM (2007) General versus specific actions of mild-moderate hypothermia in attenuating cerebral ischemic damage. J Cereb Blood Flow Metab 27(12):1879–1894. https://doi.org/10.1038/sj.jcbfm.9600540
Zhao Z, Ren Y, Jiang H, Huang Y (2021) Dexmedetomidine inhibits the PSD95-NMDA receptor interaction to promote functional recovery following traumatic brain injury. Exp Ther Med 21(1):4. https://doi.org/10.3892/etm.2020.9436
Zhou X, Zhang T, Wu J (2019) Brimonidine enhances inhibitory postsynaptic activity of OFF- and ON-type retinal ganglion cells in a Wistar rat chronic glaucoma model. Exp Eye Res 189:107833. https://doi.org/10.1016/j.exer.2019.107833
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments. This work was supported by the National Natural Science Foundation of China (grant numbers : 82073824 and 81100873) and Yichang Medical and Health Research Project (grant number: A22-2-068).
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: 82073824 and 81100873) and Yichang Medical and Health Research Project (grant number: A22-2–068).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jing Chen, Juan Zhang and Dan-Dan Yang. The first draft of the manuscript was written by Jing Chen and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the National Institutes of Health guidelines. All animal experiments were approved by the Ethics Committee of China Three Gorges University (No.00277751).
Consent to participate
Not Applicable. This article does not contain any studies with human participants performed by any of the authors.
Consent for publication
Not Applicable. This article does not contain any studies with human participants performed by any of the authors.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jing Chen, Juan Zhang and Dan-Dan Yang co-first author.
Appendix
Appendix
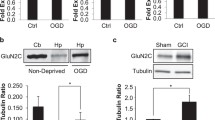
Figure 8
Rights and permissions
About this article
Cite this article
Chen, J., Zhang, J., Yang, DD. et al. Clonidine ameliorates cerebral ischemia–reperfusion injury by up-regulating the GluN3 subunits of NMDA receptor. Metab Brain Dis 37, 1829–1841 (2022). https://doi.org/10.1007/s11011-022-01028-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-022-01028-y