Abstract
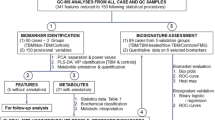
We report the potential role of 1H Nuclear Magnetic Resonance (NMR) based metabolomics in tuberculous meningitis (TBM). We also correlate the significant metabolites with clinical-radiological parameters. Forty-three patients with TBM were included, and their severity of meningitis was graded as stages I to III, and patients with positive Mycobacterium tuberculosis or its nucleic acid was considered as definite TBM. 1H NMR-based metabolomic study was performed on (CSF) samples, and the significant metabolites compared to healthy controls were identified. Outcome at three months was defined as death, poor and good based on the modified Rankin Scale. These metabolites were compared between definite and probable groups of TBM, and also correlated with MRI findings. About 11 metabolites were found to be significant for distinguishing TBM from the controls. In TBM, lactate, glutamate, alanine, arginine, 2-hydroxyisobutyrate, formate, and cis-aconitate were upregulated, and glucose, fructose, glutamine, and myo-inositol were downregulated compared to the controls. For differentiating TBM from the controls, the AUC of the ROC curve generated using these significant metabolites was 0.99, with a 95% confidence interval from 0.96 to 1, demonstrating that these metabolites were able to classify cases with good sensitivity and specificity. Lactate concentration in CSF correlated with hemoglobin, CSF glucose, and infarction. The outcome did not correlate with metabolomics parameters. NMR-based CSF metabolomics have a potential role in differentiating TBM from the controls.





Similar content being viewed by others
Availability of data and material
The data will be available at a reasonable request by the author.
References
Al-Mubarak R, Vander Heiden J, Broeckling CD, Balagon M, Brennan PJ, Vissa VD (2011) Serum metabolomics reveals higher levels of polyunsaturated fatty acids in lepromatous leprosy: potential markers for susceptibility and pathogenesis. PLoS Negl Trop Dis 5(9):e1303
Budczies J, Denkert C, Müller BM, Brockmöller SF, Klauschen F, Györffy B, Dietel M, Richter-Ehrenstein C, Marten U, Salek RM, Griffin JL, Hilvo M, Orešič M, Wohlgemuth G, Fiehn O (2012) Remodeling of central metabolism in invasive breast cancer compared to normal breast tissue - a GC-TOFMS based metabolomics study. BMC Genomics 13:334
Chatterji T, Singh S, Sen M, Singh AK, Agarwal GR, Singh DK, Srivastava JK, Singh A, Srivastava RN, Roy R (2017) Proton NMR metabolic profiling of CSF reveals distinct differentiation of meningitis from negative controls. Clinica Chimica Acta; Int J Clin Chem 469:42–52
Coen M, O'Sullivan M, Bubb WA, Kuchel PW, Sorrell T (2005) Proton nuclear magnetic resonance-based metabonomics for rapid diagnosis of meningitis and ventriculitis. Clin Infect Diseases: Off Publ Infect Diseases Soc Am 41(11):1582–1590
DeFeo EM, Wu CL, McDougal WS, Cheng LL (2011) A decade in prostate cancer: from NMR to metabolomics. Nature reviews. Urology 8(6):301–311
Do KQ, Lauer CJ, Schreiber W, Zollinger M, Gutteck-Amsler U, Cuénod M, Holsboer F (1995) Gamma-Glutamylglutamine and taurine concentrations are decreased in the cerebrospinal fluid of drug-naive patients with schizophrenic disorders. J Neurochem 65(6):2652–2662
Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam MMV, Kastenmüller G, Adamski J, Tuomi T, Lyssenko V, Groop L, Gall WE (2013) Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 62(5):730–1737
Gao H, Dong B, Jia J, Zhu H, Diao C, Yan Z, Huang Y, Li X (2012) Application of ex vivo (1)H NMR metabolomics to the characterization and possible detection of renal cell carcinoma metastases. J Cancer Res Clin Oncol 138(5):753–761
Guo K, Bamforth F, Li L (2011) Qualitative metabolome analysis of human cerebrospinal fluid by 13C-/12C-isotope dansylation labeling combined with liquid chromatography Fourier transform ion cyclotron resonance mass spectrometry. J Am Soc Mass Spectrom 22(2):339–347
Hornig CR, Dorndorf W, Die Bedeutung des Laktatspiegels, der Lysozymkonzentration und der Phosphohexose-Isomerase-Aktivität im zerebrospinalen Liquor für die (1985) Differentialdiagnose der meningitis [significance of lactate level, lysozyme concentration and phosphohexose isomerase activity in the cerebrospinal fluid in the differential diagnosis of meningitis]. Fortschr Neurol Psychiatr 53(11):410–414
Jiménez B, Mirnezami R, Kinross J, Cloarec O, Keun HC, Holmes E, Goldin RD, Ziprin P, Darzi A, Nicholson JK (2013) 1H HR-MAS NMR spectroscopy of tumor-induced local metabolic "field-effects" enables colorectal cancer staging and prognostication. J Proteome Res 12(2):959–968
Kalita J, Misra UK, Dubey AK (2019) Role of oxidative stress in Tuberculous Meningitis: a Clinico-Radiological correlation. J Mol Neurosci 68(2):287–294
Kalita J, Misra UK, Nair PP (2009) Predictors of stroke and its significance in the outcome of tuberculous meningitis. J Stroke Cerebrovascular Diseases: Off J National Stroke Assoc 18(4):251–258
Kalita J, Misra UK, Prasad S, Bhoi SK (2014) Safety and efficacy of levofloxacin versus rifampicin in tuberculous meningitis: an open-label randomized controlled trial. J Antimicrob Chemother 69(8):2246–2251
Kalita J, Misra UK, Ranjan P (2007) Predictors of long-term neurological sequelae of tuberculous meningitis: a multivariate analysis. Eur J Neurol 14(1):33–37
Leen WG, Willemsen MA, Wevers RA, Verbeek MM (2012) Cerebrospinal fluid glucose and lactate: age-specific reference values and implications for clinical practice. PLoS One 7(8):e42745
Leib SL, Boscacci R, Gratzl O, Zimmerli W (1999) Predictive value of cerebrospinal fluid (CSF) lactate level versus CSF/blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clin Infect Diseases: Off Public Infect Diseases Soc Am 29(1):69–74
Li Z, Du B, Li J, Zhang J, Zheng X, Jia H, Xing A, Sun Q, Liu F, Zhang Z (2017) Cerebrospinal fluid metabolomic profiling in tuberculous and viral meningitis: screening potential markers for differential diagnosis. Clinica Chimica Acta; Int J Clin Chem 466:38–45
Malinski T (2007) Nitric oxide and nitroxidative stress in Alzheimer's disease. J Alzheimers Dis 11(2):207–218
Mandal R, Guo AC, Chaudhary KK, Liu P, Yallou FS, Dong E, Aziat F, Wishart DS (2012) Multi-platform characterization of the human cerebrospinal fluid metabolome: a comprehensive and quantitative update. Genome Med 4(4):38
Marais S, Thwaites G, Schoeman JF, Török ME, Misra UK, Prasad K, Donald PR, Wilkinson RJ, Marais BJ (2010) Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 10(11):803–812
Mason S, van Furth AM, Mienie LJ, Engelke UF, Wevers RA, Solomons R, Reinecke, C. J (2015) A hypothetical astrocyte-microglia lactate shuttle derived from a 1H NMR metabolomics analysis of cerebrospinal fluid from a cohort of south African children with tuberculous meningitis. Metabolomics: Off J Metab Soc 11(4):822–837
Mason S, Reinecke CJ, Solomons R, Wevers RA, Engelke UF (2017) 1H NMR spectral identification of medication in cerebrospinal fluid of pediatric meningitis. J Pharm Biomed Anal 143(5):56–61
Misra UK, Kalita J, Maurya PK (2011) Stroke in tuberculous meningitis. J Neurol Sci 303(1–2):22–30
Molina JA, Jiménez-Jiménez FJ, Gomez P, Vargas C, Navarro JA, Ortí-Pareja M, Gasalla T, Benito-León J, Bermejo F, Arenas J (1997) Decreased cerebrospinal fluid levels of neutral and basic amino acids inpatients with Parkinson's disease. J Neurol Sci 150(2):123–127
Perry TL (1982) Normal cerebrospinal fluid and brain glutamate levels in schizophrenia do not support the hypothesis of glutamatergic neuronal dysfunction. Neurosci Lett 28(1):81–85
Pieragostino D, D'Alessandro M, di Ioia M, Rossi C, Zucchelli M, Urbani A, Di Ilio C, Lugaresi A, Sacchetta P, Del Boccio P (2015) An integrated metabolomics approach for the research of new cerebrospinal fluid biomarkers of multiple sclerosis. Mol BioSyst 11(6):1563–1572
Pirisino R, Ghelardini C, De Siena G, Malmberg P, Galeotti N, Cioni L, Banchelli G, Raimondi L (2005) Methylamine: a new endogenous modulator of neuron firing? Med Sci Monitor: Int Med J Exp Clin Res 11(8):RA257–RA261
Ratuszny D, Sühs KW, Novoselova N, Kuhn M, Kaever V, Skripuletz T, Pessler F, Stangel M (2019) Identification of cerebrospinal fluid metabolites as biomarkers for Enterovirus meningitis. Int J Mol Sci 20(2):337
Raviglione MC, Snider DE Jr, Kochi A (1995) Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA 273(3):220–226
Stoop MP, Coulier L, Rosenling T, Shi S, Smolinska AM, Buydens L, Ampt K, Stingl C, Dane A, Muilwijk B, Luitwieler RL, SillevisSmitt PA, Hintzen RQ, Bischoff R, Wijmenga SS, Hankemeier T, van Gool AJ, Luider TM (2010) Quantitative proteomics and metabolomics analysis of normal human cerebrospinal fluid samples. Mol Cell Proteomics: MCP 9(9):2063–2075
STREPTOMYCIN (1948) Treatment of tuberculous meningitis. Lancet 1(6503):582–596
Sweatt AJ, Wood M, Suryawan A, Wallin R, Willingham MC, Hutson SM (2003) Branched-chain amino acid catabolism: unique segregation of pathway enzymes in organ systems and peripheral nerves. Am J Physiol Endocrinol Metab 286:E64–E76
Subramanian A, Gupta A, Saxena S, Gupta A, Kumar R, Nigam A, Kumar R, Mandal SK, Roy R (2005) Proton MR CSF analysis and a new software as predictors for the differentiation of meningitis in children. NMR Biomed 18(4):213–225
Thwaites GE, Simmons CP, Than Ha Quyen N, Thi Hong Chau T, Phuong Mai P, Thi Dung N, Hoan Phu N, White NP, Tinh Hien T, Farrar JJ (2003) Pathophysiology and prognosis in vietnamese adults with tuberculous meningitis. J Infect Dis 188(8):1105–1115
Thwaites GE, Tran TH (2005) Tuberculous meningitis: many questions, too few answers. Lancet Neurol 4(3):160–170
Thwaites GE, van Toorn R, Schoeman J (2013) Tuberculous meningitis: more questions, still too few answers. Lancet Neurol 12(10):999–1010
Tsai G, van Kammen DP, Chen S, Kelley ME, Grier A, Coyle JT (1998) Glutamatergic neurotransmission involves structural and clinical deficits of schizophrenia. Biol Psychiatry 44(8):667–674
Vadnal R, Parthasarathy L, Parthasarathy R (1997) Role of inositol in the treatment of psychiatric disorders. CNS Drugs 7(1):6–16
Van Well GT, Paes BF, Terwee CB, Springer P, Roord JJ, Donald PR, van Furth AM, Schoeman JF (2009) Twenty years of pediatric tuberculous meningitis: a retrospective cohort study in the western cape of South Africa. Pediatrics 123(1):e1–e8
Wevers RA, Engelke U, Wendel U, de Jong JG, Gabreëls FJ, Heerschap A (1995) Standardized method for high-resolution 1H-NMR of cerebrospinal fluid. Clin Chem 41(5):744–751
Wishart DS, Lewis MJ, Morrissey JA, Flegel MD, Jeroncic K, Xiong Y, Cheng D, Eisner R, Gautam B, Tzur D, Sawhney S, Bamforth F, Greiner R, Li L (2008) The human cerebrospinal fluid metabolome. Journal of chromatography. B, Analytical Technol Biomed Life Sci 871(2):164–173
World Health Organization (2016) Global Tuberculosis Report 21st edition
Xu Y, Ringgaard S, Mariager CØ, Bertelsen LB, Schroeder M, Qi H, Laustsen C, Stødkilde-Jørgensen H (2017) Hyperpolarized 13C magnetic resonance imaging can detect metabolic changes characteristic of penumbra in ischemic stroke. Tomography (Ann Arbor, Mich. 3(2):67–73
Yi J, Horky LL, Friedlich AL, Shi Y, Rogers JT, Huang X (2009) L-arginine and Alzheimer's disease. Int J Clin Exp Pathol 2(3):211–238
Zimmermann M, Kogadeeva M, Gengenbacher M, McEwen G, Mollenkopf HJ, Zamboni N, Kaufmann S, Sauer U (2017) Integration of metabolomics and Transcriptomics reveals a complex diet of mycobacterium tuberculosis during early macrophage infection. mSystems 2(4):e00057–e00017
Acknowledgments
We thank Mr. Shakti Kumar for his secretarial help.
Funding support
This work was supported by an Intramural Grant (A-04-PGI/IMP/76/2018) of Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Sanctioned to Professor Jayantee Kalita.
Author information
Authors and Affiliations
Contributions
RP: NMR experiment and data analysis, RS: Data collection and analysis of data, BB: Supervising the experimental data and their result, and writing the manuscript. JK: management, clinical data collection, interpretation of data, writing and drafting the manuscript. RH: Provided control CSF sample. UKM: Supervision, patient management, and writing the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This study was ethically approved by the institutional ethics committee, SGPGIMS, Lucknow, India (2018–28-IMP-102, dated 13-4-2018).
Consent to participate/ informed consent
Informed consent to participate in the study must be obtained from participants.
Informed consent
Informed consent was obtained from all individual participants or from their first-degree relatives.
Consent for publication
The participant has consented to the submission of the journal.
Conflicts of interest/competing interests
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Supplementary Fig. 1.
A) PCA 2D score plot of TBM (Definite vs. Probable) displaying significant overlap as both are disease subgroup, B) PLS-DA score plot of TBM (Definite vs. Probable), C) VIP plot of TBM (Definite vs. Probable, D) PLS-DA loading plot of TBM (Definite vs. Probable). Lactate and glucose are the most important metabolites for discrimination, followed by glutamate, glutamine, o-phosphoserine, arginine, and malonate. (TIFF 254 kb) (PNG 6534 kb)

Supplementary Fig. 2.
A) PCA 2D score plot showing A) control vs. TBM, B) control Vs. definite, and C) Control vs. probable. There is clear clustering between the two groups; the best separation is observed between control vs. definite TBM. (PNG 14202 kb)

Supplementary Fig. 3.
Stack plot of a series of 1H1D spectra(cpmg). Panels a-i are expanded sections from control CSF, and panels j-s are from definite TBM cases. Similar observations in dd at 4.45 is shown with dotted box were also observed. The identity of this spin system could not be confirmed from the databases. In one case panel, 'r' a quartet at 4.43 was observed, which may be from acetoin- an external energy store for bacteria. This peak could not be detected in other samples. (PNG 2991 kb)

Supplementary Fig. 4.
Panels A-F has expended portions of the various regions of the tilted J-RES spectrum. Numbers near each peak position represent metabolites from the metabolites list in supplementary table 1. (PNG 2751 kb)


Supplementary Fig. 6.
(A) Full 1H-1H TOCSY spectrum, (B) Expended portion of the 1H-1H TOCSY spectrum showing the important region that was assigned. Individual peak assignments are further displayed in supplementary Figs 11-13. (C) Expanded portion showing the cross-peaks between the peak at 4.45 with 1.75 and 0.88 ppm. Half of the TBM cases displayed this peak at 4.45, while no such peak was observed in the control group. (PNG 1426 kb)

Supplementary Fig. 7.
Expended portion for each peak from 1H-13C HSQC spectrum that was assigned. Numbers represent metabolites from supplementary table 1. (PNG 1028 kb)

Supplementary Fig. 8.
Expended portion for each peak from 1H-13C HSQC spectrum that was assigned. Numbers represent metabolites from supplementary table 1. (PNG 1078 kb)

Supplementary Fig. 9.
Expended portion for each peak from 1H-13C HSQC spectrum that was assigned. Numbers represent metabolites from supplementary table 1. (PNG 1153 kb)

Supplementary Fig. 10.
Expended portion for each peak from 1H-13C HSQC spectrum that was assigned. Numbers represent metabolites from supplementary table 1. (PNG 950 kb)

Supplementary Fig. 11.
Expended portion for each peak from 1H-1H TOCSY spectrum that was assigned. Numbers represent metabolites from supplementary table 1. (PNG 1174 kb)

Supplementary Fig. 12.
Expended portion for each peak from 1H-1H TOCSY spectrum that was assigned. Numbers represent metabolites from supplementary table 1. (PNG 784 kb)

Supplementary Fig. 13.
Expended portion for each peak from 1H-1H TOCSY spectrum that was assigned. Numbers represent metabolites from supplementary table 1. (PNG 1206 kb)
ESM 14
(PDF 677 kb)
Rights and permissions
About this article
Cite this article
Parihar, R., Shukla, R., Baishya, B. et al. NMR based CSF metabolomics in tuberculous meningitis: correlation with clinical and MRI findings. Metab Brain Dis 37, 773–785 (2022). https://doi.org/10.1007/s11011-021-00860-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00860-y