Abstract
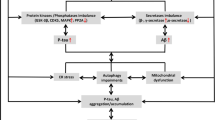
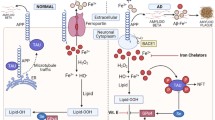
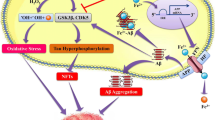
Metal homeostasis in the central nervous system (CNS) is a crucial component of healthy brain function, because metals serve as enzymatic cofactors and are key components of intra- and inter-neuronal signaling. Metal dysregulation wreaks havoc on neural networks via induction and proliferation of pathological pathways that cause oxidative stress, synaptic impairment, and ultimately, cognitive deficits. Thus, exploration of metal biology in relation to neurodegenerative pathology is essential in pursuing novel therapies for Alzheimer’s Disease and other neurodegenerative disorders. This review covers mechanisms of action of aluminum, iron, copper, and zinc ions with respect to the progressive, toxic accumulation of extracellular β-amyloid plaques and intracellular hyperphosphorylated neurofibrillary tau tangles that characterizes Alzheimer’s Disease, with the goal of evaluating the therapeutic potential of metal ion interference in neurodegenerative disease prevention and treatment. As neuroscientific interest in the role of metals in neurodegeneration escalates—in large part due to emerging evidence substantiating the interplay between metal imbalances and neuropathology—it becomes clear that the use of metal chelating agents may be a viable method for ameliorating Alzheimer’s Disease pathology, as its etiology remains obscure. We conclude that, although metal therapies can potentially deter neurodegenerative processes, the most promising treatments will remain elusive until further understanding of neurodegenerative etiology is achieved. New research directions may best be guided by animal models of neurodegeneration, which reveal specific insights into biological mechanisms underlying dementia.



Similar content being viewed by others
Data availability
Datasets from this study are available from the corresponding author on reasonable request.
References
2020 Alzheimer’s disease facts and figures (2020) Alzheimers dement https://doi.org/10.1002/alz.12068
Abbaspour N, Hurrell R, Kelishadi R (2014) Review on iron and its importance for human health. J Res Med Sci 19:164–174
Ashraf A, Clark M, So PW (2018) The Aging of Iron Man. Front Aging Neurosci 10:65. https://doi.org/10.3389/fnagi.2018.00065
Ashraf A, So PW (2020) Spotlight on ferroptosis: iron-dependent cell death in Alzheimer’s disease. Front Aging Neurosci 12:196. https://doi.org/10.3389/fnagi.2020.00196
Ayton S, Wang Y, Diouf I, Schneider JA, Brockman J, Morris MC, Bush AI (2020) Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol Psychiatry 25:2932–2941. https://doi.org/10.1038/s41380-019-0375-7
Bagheri S, Aghaei H, Ghaedi M, Asfaram A, Monajemi M, Bazrafshan AA (2018) Synthesis of nanocomposites of iron oxide/gold (Fe3O4/Au) loaded on activated carbon and their application in water treatment by using sonochemistry: optimization study. Ultrason Sonochem 41:279–287. https://doi.org/10.1016/j.ultsonch.2017.09.031
Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol 10:819–828. https://doi.org/10.1016/S1474-4422(11)70072-2
Barnham KJ, Bush AI (2014) Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem Soc Rev 43:6727–6749. https://doi.org/10.1039/c4cs00138a
Berg D, Youdim MB (2006) Role of iron in neurodegenerative disorders. Top Magn Reson Imaging 17:5–17. https://doi.org/10.1097/01.rmr.0000245461.90406.ad
Bharathi Shamasundar NM, Sathyanarayana Rao TS, Dhanunjaya Naidu M, Ravid R, Rao KS (2006) A new insight on Al-maltolate-treated aged rabbit as Alzheimer’s animal model. Brain Res Rev 52:275–292. https://doi.org/10.1016/j.brainresrev.2006.04.003
Bush AI (2008) Drug development based on the metals hypothesis of Alzheimer’s disease. J Alzheimers Dis 15:223–240. https://doi.org/10.3233/jad-2008-15208
Chen WT, Liao YH, Yu HM, Cheng IH, Chen YR (2011) Distinct effects of Zn2+, Cu2+, Fe3+, and Al3+ on amyloid-β stability, oligomerization, and aggregation: amyloid-β destabilization promotes annular protofibril formation. The Journal of Biological Chemistry 286(11):9646–9656
Chevion M, Korbashi P, Katzhandler J, Saltman P (1990) Zinc–a redox-inactive metal provides a novel approach for protection against metal-mediated free radical induced injury: study of paraquat toxicity in E. coli. Adv Exp Med Biol 264:217–222. https://doi.org/10.1007/978-1-4684-5730-8_35
Cristovao JS, Santos R, Gomes CM (2016) Metals and neuronal metal binding proteins implicated in Alzheimer’s disease. Oxid Med Cell Longev 2016:9812178. https://doi.org/10.1155/2016/9812178
Cuajungco MP, Lees GJ (1997) Zinc and Alzheimer’s disease: Is there a direct link? Brain Res Brain Res Rev 23:219–236. https://doi.org/10.1016/s0165-0173(97)00002-7
Date AA, Hanes J, Ensign LM (2016) Nanoparticles for oral delivery: design, evaluation and state-of-the-art. J Control Release 240:504–526. https://doi.org/10.1016/j.jconrel.2016.06.016
Derry PJ, Hegde ML, Jackson GR, Kayed R, Tour JM, Tsai AL, Kent TA (2020) Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. Prog Neurobiol 184:101716. https://doi.org/10.1016/j.pneurobio.2019.101716
Duce JA et al (2010) Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 142:857–867. https://doi.org/10.1016/j.cell.2010.08.014
Exley C, Mold MJ (2019) Aluminium in human brain tissue: how much is too much? J Biol Inorg Chem 24:1279–1282. https://doi.org/10.1007/s00775-019-01710-0
Frota NAF, Caramelli P, Barbosa ER (2009) Cognitive impairment in Wilson’s disease. Dement Neuropsychol 3:16–21. https://doi.org/10.1590/S1980-57642009DN30100004
Gaggelli E, Kozlowski H, Valensin D, Valensin G (2006) Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev 106:1995–2044. https://doi.org/10.1021/cr040410w
Garcia T, Esparza JL, Nogues MR, Romeu M, Domingo JL, Gomez M (2010) Oxidative stress status and RNA expression in hippocampus of an animal model of Alzheimer’s disease after chronic exposure to aluminum. Hippocampus 20:218–225. https://doi.org/10.1002/hipo.20612
Goedert M, Spillantini MG (2006) A century of Alzheimer’s disease. Science 314:777–781. https://doi.org/10.1126/science.1132814
Ha C, Ryu J, Chan BP (2007) Metal ions differentially influence the aggregation and deposition of Alzheimer's β-amyloid on a solid template. Biochemistry 46(20)6118–6125
Henning HV (1989) [Aluminum toxicity] Klin Wochenschr 67:1221–1228 https://doi.org/10.1007/BF01745293
Hinkebein JH, Martin TA, Callahan CD, Johnstone B (2003) Traumatic brain injury and Alzheimer’s: deficit profile similarities and the impact of normal ageing. Brain Inj 17:1035–1042. https://doi.org/10.1080/0269905031000110490
Hosovski E, Mastelica Z, Sunderic D, Radulovic D (1990) Mental abilities of workers exposed to aluminium. Med Lav 81:119–123
Igbokwe IO, Igwenagu E, Igbokwe NA (2019) Aluminium toxicosis: a review of toxic actions and effects. Interdiscip Toxicol 12:45–70. https://doi.org/10.2478/intox-2019-0007
Jellinger KA (2013) The relevance of metals in the pathophysiology of neurodegeneration, pathological considerations. Int Rev Neurobiol 110:1–47. https://doi.org/10.1016/B978-0-12-410502-7.00002-8
Jucker M, Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501:45–51. https://doi.org/10.1038/nature12481
Kontoghiorghes GJ, Kontoghiorghe CN (2020) Iron and chelation in biochemistry and medicine: new approaches to controlling iron metabolism and treating related diseases cells 9 https://doi.org/10.3390/cells9061456
Lang C, Letzel S (1995) Neurotoxicity of aluminum. Study of a long-term exposed sample of workers of an aluminum powder industry. Fortschr Med 113:30–31
Lidsky TI (2014) Is the Aluminum Hypothesis dead? J Occup Environ Med 56:S73-79. https://doi.org/10.1097/JOM.0000000000000063
Liu Y, Nguyen M, Robert A, Meunier B (2019) Metal ions in Alzheimer’s disease: a key role or not? Acc Chem Res 52:2026–2035. https://doi.org/10.1021/acs.accounts.9b00248
Marreiro DD, Cruz KJ, Morais JB, Beserra JB, Severo JS, de Oliveira AR (2017) Zinc and oxidative stress: current mechanisms antioxidants (Basel) 6 https://doi.org/10.3390/antiox6020024
Masaldan S, Belaidi AA, Ayton S, Bush AI (2019) Cellular senescence and iron dyshomeostasis in Alzheimer’s disease. Pharmaceuticals (Basel) 12 https://doi.org/10.3390/ph12020093
McDermott JR, Smith AI, Iqbal K, Wisniewski HM (1979) Brain aluminum in aging and Alzheimer disease. Neurology 29:809–814. https://doi.org/10.1212/wnl.29.6.809
Meloni G, Sonois V, Delaine T et al. (2008) Metal swap between Zn7-metallothionein-3 and amyloid-β-Cu protects against amyloid-β toxicity. Nature Chemical Biology 4(6):366–372
Morris GP, Clark IA, Vissel B (2014) Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol Commun 2:135. https://doi.org/10.1186/s40478-014-0135-5
Mujika JI, Ruiperez F, Infante I, Ugalde JM, Exley C, Lopez X (2011) Pro-oxidant activity of aluminum: stabilization of the aluminum superoxide radical ion. J Phys Chem A 115:6717–6723. https://doi.org/10.1021/jp203290b
Mullane K, Williams M (2013) Alzheimer’s therapeutics: continued clinical failures question the validity of the amyloid hypothesis-but what lies beyond? Biochem Pharmacol 85:289–305. https://doi.org/10.1016/j.bcp.2012.11.014
Nandi A, Yan LJ, Jana CK, Das N (2019) Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid Med Cell Longev 2019:9613090. https://doi.org/10.1155/2019/9613090
Oteiza PI (2012) Zinc and the modulation of redox homeostasis. Free Radic Biol Med 53:1748–1759. https://doi.org/10.1016/j.freeradbiomed.2012.08.568
Pal A, Kumar A, Prasad R (2014) Predictive association of copper metabolism proteins with Alzheimer’s disease and Parkinson’s disease: a preliminary perspective. Biometals 27:25–31. https://doi.org/10.1007/s10534-013-9702-7
Ribes D, Torrente M, Vicens P, Colomina MT, Gomez M, Domingo JL (2012) Recognition memory and beta-amyloid plaques in adult Tg2576 mice are not modified after oral exposure to aluminum. Alzheimer Dis Assoc Disord 26:179–185. https://doi.org/10.1097/WAD.0b013e3182211ab1
Savory J, Rao JK, Huang Y, Letada PR, Herman MM (1999) Age-related hippocampal changes in Bcl-2: Bax ratio, oxidative stress, redox-active iron and apoptosis associated with aluminum-induced neurodegeneration: increased susceptibility with aging. Neurotoxicology 20:805–817
Scheiber IF, Mercer JF, Dringen R (2014) Metabolism and functions of copper in brain. Prog Neurobiol 116:33–57. https://doi.org/10.1016/j.pneurobio.2014.01.002
Squitti R, Polimanti R (2013) Copper phenotype in Alzheimer’s disease: Dissecting the pathway. Am J Neurodegener Dis 2:46–56
Suh SW, Jensen KB, Jensen MS, Silva DS, Kesslak PJ, Danscher G, Frederickson CJ (2000) Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res 852:274–278. https://doi.org/10.1016/s0006-8993(99)02096-x
Sun XY, Wei YP, Xiong Y et al (2012) Synaptic released zinc promotes tau hyperphosphorylation by inhibition of Protein Phosphatase 2A (PP2A). The Journal of Biological Chemistry 287(14):11174–11182
Tolar M, Abushakra S, Sabbagh M (2020) The path forward in Alzheimer’s disease therapeutics: reevaluating the amyloid cascade hypothesis. Alzheimers Dement 16:1553–1560. https://doi.org/10.1016/j.jalz.2019.09.075
Tõugu V, Karafin A, Zovo K et al (2009) Zn(II)- and Cu(II)-induced non-fibrillar aggregates of amyloid-β (1–42) peptide are transformed to amyloid fibrils, both spontaneously and under the influence of metal chelators. J Neurochem 110(6)1784–1795
Walton JR (2007) An aluminum-based rat model for Alzheimer’s disease exhibits oxidative damage, inhibition of PP2A activity, hyperphosphorylated tau, and granulovacuolar degeneration. J Inorg Biochem 101:1275–1284. https://doi.org/10.1016/j.jinorgbio.2007.06.001
Wang CY, Wang T, Zheng W et al (2010) Zinc overload enhances APP cleavage and Aβ deposition in the Alzheimer mouse brain. PLoS ONE 5(12):e15349
Wang L, Yin Y-L, Liu X-Z et al (2020) Current understanding of metal Ions in the pathogenesis of Alzheimer’s disease. Transl Neurodegener 9:10. https://doi.org/10.1186/s40035-020-00189-z
Wisniewski HM, Wen GY (1992) Aluminium and Alzheimer’s disease. Ciba Found Symp 169:142–154; discussion 154–164 https://doi.org/10.1002/9780470514306.ch9
Yang GJ, Liu H, Ma DL, Leung CH (2019) Rebalancing metal dyshomeostasis for Alzheimer’s disease therapy. J Biol Inorg Chem 24:1159–1170. https://doi.org/10.1007/s00775-019-01712-y
Yokel RA (2000) The toxicology of aluminum in the brain: a review. Neurotoxicology 21:813–828
Yoshiike Y, Tanemura K, Murayama O et al., (2001) New insights on how metals disrupt amyloid beta-aggregation and their effects on amyloid-beta cytotoxicity. The Journal of Biological Chemistry 276(34):32293–32299
Zheng W, Aschner M, Ghersi-Egea JF (2003) Brain barrier systems: a new frontier in metal neurotoxicological research. Toxicol Appl Pharmacol 192:1–11. https://doi.org/10.1016/s0041-008x(03)00251-5
Acknowledgements
This study was supported by funding from the National Center for Toxicological Research/FDA [Protocol #763101 to SS]
Funding
This study was supported by funding from the National Center for Toxicological Research/FDA [Protocol #763101 to SS].
Author information
Authors and Affiliations
Contributions
Nikita Das (ND) is the lead author who has substantially contributed in writing the manuscript. James Raymick (JR) has significant input on histological labeling of sections, data collection. Sumit Sarkar (SS) is the corresponding author and primary input on experiment design, performed animal sacrifice, tissue processing and immune-labeling of histological sections, data collection and interpretation of photomicrographs and editing of manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All experimental procedures described here for rodent and human tissues were approved by the appropriate committees at the National Center for Toxicological Research/FDA, including the NCTR Office of Research, the Regulatory Compliance and Risk Management Director and the FDA Research Involving Human Subjects Committee (RIHSC). As determined by the FDA RIHSC, this study did not reach the definition of “Human Subject Research” at 45 CFR 46.102(f) and thus, 45 CFR Part 46 does not apply.
Conflict of interest
None of the authors in this manuscript has any conflict of interest.
Disclaimer
This manuscript reflects the views of the authors and does not necessarily reflect those of the Food and Drug Administration.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, N., Raymick, J. & Sarkar, S. Role of metals in Alzheimer’s disease. Metab Brain Dis 36, 1627–1639 (2021). https://doi.org/10.1007/s11011-021-00765-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00765-w