Abstract
Brain glucose uptake is usually reduced in type 2 diabetes owing to downregulation of brain glucose transporters. The ability of Vernonia amygdalina to stimulate glucose uptake as well as ameliorate glucose-induced oxidative stress and proinflammation were investigated in rat brain. Hot infusion of V. amygdalina leaves was incubated with rat brain tissues for 2 h in the presence of glucose. Another incubation with glucose only, served as negative control while metformin served as positive control. Incubation of brain tissues with V. amygdalina led to significant (p < 0.05) increase in glucose uptake, reduced glutathione, nitric oxide and non-thiol proteins levels, superoxide dismutase, catalase and ATPase activities, while concomitantly decrease in myeloperoxidase activity and malondialdehyde level compared to the negative control. Incubation with glucose only, led to the development of nitrate, amide II and amide I functional groups which were removed on incubation with the infusion. LC-MS analysis revealed depletion of oxidative stress-induced 2-keto-glutaramic acid and cysteinyl-tyrosine metabolites in brain tissues, with concomitant generation of S-formylglutathione and adenosine tetraphosphate by the infusion. Pathway analysis of the metabolites revealed an activation of pyruvate metabolism pathway in the negative control, with the infusion reducing the intensity fold. LC-MS analysis of the infusion revealed the presence of l–serine, l-cysteine, l-proline, nicotinic acid, cumidine, salicylic acid, isoquinoline, 3-methyl-, and γ-octalactone. Except for l–serine, l-cysteine and l-proline, the other compounds were predicted to be permeable across the blood brain barrier. These results indicate the brain glucose uptake stimulatory and neuroprotective effect of V. amygdalina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) has been recognized as a global epidemic affecting over 425 million people in 2017, with an estimated 48% increase postulated for 2045 (I.D.F. 2018). It is a metabolic disorder characterized by high blood glucose (hyperglycemia) owing to failure of the pancreatic β cell to secrete insulin and/or inability of the cells to utilize secreted insulin as seen in type 1 diabetes (T1D) and type 2 diabetes (T2D) respectively (Erukainure et al. 2017a). T2D accounts for more than 90% of all diabetes types, thus making it a major contributor to diabetic morbidity and mortality (I.D.F. 2018). Increased production of reactive oxygen species (ROS) have been reported in extreme hyperglycemia in T2D (Erukainure et al. 2018; Maritim et al. 2003). Inability of the tissues’ endogenous system to mop these free radicals, results in oxidative stress which has been implicated in the pathogenesis and progression of microvascular and macrovascular complications in T2D (Whitlow et al. 2015). Several recent studies reported that T2D is one of the major culprits in inducing microvascular complications in brain (Vagelatos and Eslick 2013; Whitlow et al. 2015; Wrighten et al. 2009). This has been attributed to alteration in insulin signaling and glucose homeostasis in the CNS (Wrighten et al. 2009). Glucose is the predominant source of energy in the brain and is transported across the blood brain barrier (BBB) by glucose transporters (McEwen and Reagan 2004; Reagan et al. 2008; Wrighten et al. 2009). These transporters are often down regulated in T2D, thus impairing glucose uptake in the brain (Gejl et al. 2017; Hwang et al. 2017; Pardridge et al. 1990).
The neuroprotective effects of medicinal plants have been reported in a number of recent studies (Aslam and Sial 2014; Mohebbatia et al. 2017; Pandit 2011; Uddin et al. 2013). These effects have been attributed to the phytochemical constituents of the plants, particularly the phenolics with reported antioxidant, antidiabetic and ability to transverse the BBB (Rice-Evans et al. 1997; Saravanan and Parimelazhagan 2014; Youdim et al. 2003). Some of these plants have also been reported for their antidiabetic properties, indicating their protective potential against diabetic brain degeneration. Amongst such plants is Vernonia amygdalina.
Vernonia amygdalina is amongst the most studied plants for its medicinal properties. It is a leafy vegetable regarded as bitter leaf owing to its bitter taste. It is consumed as a food and employed in traditional medicine in the treatment of various ailments including diabetes, hypertension, and infertility (Farombi and Owoeye 2011; Ijeh and Ejike 2011). Its folkloric medicinal claims have been authenticated by several studies which includes anti-obesogenic (Adaramoye et al. 2008), antioxidant (Adesanoye and Farombi 2010; Iwalokun et al. 2006), anticancer (Howard et al. 2003; Yedjou et al. 2008), antidiabetes (Ong et al. 2011; Saliu et al. 2012), anti-sickling (Afolabi et al. 2012; Chikezie 2006) and antihypertension (Ajibola et al. 2011; Saliu et al. 2012). Its protective effects against brain degeneration as well as in improving learning have also been reported (Ebuehi and Ajagun-Ogunleye 2017; Farombi and Owoeye 2011; Owoeye et al. 2011). Its reported phytochemical constituents include steroid glucosides, sesquiterpene lactones, stigmastane-type and steroidal saponins, terpenes, and polyphenols (Farombi and Owoeye 2011; Ijeh and Ejike 2011; Saliu et al. 2012; Yeap et al. 2010).
Although the neuroprotective effect of V. amygdalina has been reported, there is however a dearth in its ability to stimulate glucose uptake in brain tissues and the metabolic pathways that may be involved. Hence, this study was undertaken to investigate the glucose uptake enhancing properties of V. amygdalina hot infusion, and its antioxidative and anti-proinflammatory effects in brain tissues, as well as the metabolic pathways and metabolites that may be involved. The ability of d-glucose to molecularly interact with key antioxidant and proinflammatory enzymes was also investigated in silico.
Materials and methods
Plant material
Vernonia amygdalina leaves were harvested in January 2017 at Benin city, Nigeria. They were identified, deposited and assigned the voucher number: UBHV342 at the herbarium of the Department of Botany, University of Benin, Benin city, Nigeria.
The leaves were air dried to a constant weight, blended, and stored at room temperature in zip-lock bags until further analysis.
Extraction
Blended V. amygdalina leaves sample (10 g) was infused in boiling distilled water and allowed to extract overnight. The extract was decanted and concentrated in a water bath at 50 °C to yield 4.2 g concentrate, which was stored in glass vials at 4 °C until further analysis.
A stock solution of 1 mg/mL was prepared with distilled water. Different concentrations consisting of 15, 30, 60, 120 and 240 μg/mL were prepared from the stock solution for the study.
Animals
Five male albino rats (Sprague Dawley strain; 200–250 g) were obtained from the Biomedical Research Unit (BRU), University of KwaZulu-Natal, Durban, South Africa. They were humanely sacrificed by euthanizing with halothane after overnight fasting. Their brains were collected for ex vivo studies.
The animals were maintained in accordance with the approved guidelines of the animal ethics committee of the University of KwaZulu-Natal, Durban, South Africa (protocol approval number: AREC/020/017D).
Glucose uptake in isolated rat brain
This was carried out by incubating 0.6 g of the fresh harvested brains with 8 mL of Krebs buffer containing 11.1 mM glucose and different V. amygdalina infusion concentrations under a 5%CO2, 95% oxygen and 37 °C conditions for 2 h (Chukwuma and Islam 2015). An incubation without the infusion, served as a control. Metformin (Pharmed Ltd., Durban, South Africa) was used as the standard antidiabetic drug.
At the end of incubation, the brain tissues were removed and homogenized in cold phosphate buffer (50 mM, pH 7.5) with triton X-100. The homogenized tissues were centrifuged at 15,000 rpm for 10 mins at 4 °C, and supernatants stored at −20 °C until further analysis.
All chemicals used were purchased from Sigma-Aldrich, South Africa unless otherwise indicated in manuscript.
Determination of glucose uptake
Glucose concentrations of the buffer were measured before and after the incubation with an automated chemistry analyzer (Labmax Plenno, Labtest Inc., Lagoa Santa, Brazil). Glucose uptake was then calculated using following formula:
Where GC1 and GC2 are glucose concentrations (mg/dL) before and after incubation, respectively.
Determination of oxidative stress and proinflammation parameters
The homgenized tissues were assayed for their reduced glutathione (GSH) (Ellman 1959), Nitric Oxide (NO) (Tsikas 2005), non-protein thiol (Adefegha et al. 2017; Habig et al. 1974) malondialdehyde (MDA) (Chowdhury and Soulsby 2002) levels, catalase (Chance and Maehly 1955), myeloperoxidase (Granell et al. 2003), superoxide dismutase (SOD) (Kakkar et al. 1984) and ATPase (Adewoye et al. 2000) activities.
Molecular docking
Molecular docking was carried out to determine the binding potential of D-glucose to ATPase, catalase and myeloperoxidase. 3D crystal structures of ATPase, catalase, and myeloperoxidase with PDB access code: 4HYT (Laursen et al. 2013), 1TBU (Yang et al. 2013), and 1DNW (Blair-Johnson et al. 2001) respectively were retrieved from protein data bank. The protein resolutions were 3.4 Å, 2.2 Å, and 1.9 Å respectively. AutoDock tools (Sanner1999) 1.5.4 was used to determine the suitable grid box size for the potential binding site. The structure of D-glucose was retrieved from PUBMED and optimized using Gaussian 09,(Frisch et al.2009) This was done to obtain minimized confromation. The determined dimension was X = 40 Y = 40 Z = 40 with 1.00 Å as the grid spacing. Lamarckian genetic algorithm method was applied to obtain optimum binding site for the ligand. (Yang et al. 2013) Gasteiger charges were computed using the AutoDock Tools graphical user interface supplied by MGL Tools (Morris et al. 2009).
Metabolic profiling
Metabolites were extracted from the tissue homogenates using the method as described previously (Chan et al. 2013) with slight modifications (Erukainure et al. 2017b).
The extracted metabolites were scanned on Fourier-transform infrared (FT-IR) spectrophotometer (Perkin Elmer Spectrum 400) at room temperature (25–28 °C) at 300–4000 cm−1 spectral range. The chemical functional groups were determined by comparing the peak heights and shifts to the IR spectroscopy correlation table.
The metabolic constituents of were determined by subjecting the extracted metabolites to mass spectrometry by Liquid Chromatography – Mass Spectroscopy (LC-MS; Shimadzu LCMS-2020). Direct search of mass spectral data against the Human Metabolome Database (HMDB) was used in identifying metabolites (Wishart et al. 2012).
MetaboAnalyst 4.0 was used in analyzing pathways of significantly altered brain metabolites as described pereviously (Xia and Wishart 2016).
Determination of the phytochemical constituents of V. amygdalina hot infusion
The phytochemical constituents of the infused extract of V. amygdalina were determined via LC-MS analysis as described by Erukainure et al. (2017b). The compounds were identified using the NIST library online.
In silico prediction of BBB permeability and oral lethal dose toxicity
In silico prediction of BBB permeability and oral lethal dose toxicity of the LC-MS identified compounds of V. amygdalina in comparison with their qualitative structure activity relationship (QSAR) and virtual molecular structure activity relationship studies (SARs) were determined using pkCSM – pharmacokinetics (Pires et al. 2015) and PROTOX (Drwal et al. 2014) servers.
Statistics
Statistical significance was determined using one-way analysis of variance (ANOVA), with results presented as mean ± SD. Significant difference was established at p < 0.05. Statistical analyses were carried out using IBM SPSS for Windows, version 23.0 (SPSS Inc., Chicago, IL).
Results
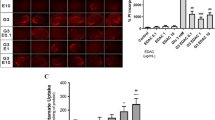
Incubation of brain tissues in V. amygdalina infusion in the presence of D-glucose, led to significant (p < 0.05) increase in glucose uptake compared to the control (glucose only) and metformin as depicted in Fig. 1. The uptake was dose dependent, with the highest concentration showed the highest uptake. Metformin showed little or no significant effect on glucose uptake.
Incubation of brain tissues with D-glucose significantly (p < 0.05) depleted GSH and NTP level, SOD and catalase activities, with concomitant increased levels of MDA as depicted in Fig. 2a–e. These were significantly (p < 0.05) reversed on incubation with V. amygdalina and metformin respectively, depicting an antioxidative effect.
Effect of V. amygdalina infusion on (a) GSH level; (b) SOD and (c) catalase activities; and (d) MDA levels in glucose treated brain. Data = mean + SD; n = 3. *Statistically significant compared to Glucose-only treated tissues. Data = mean ± SD; n = 3. *Statistically significant compared to Glucose-only treated tissues; #Statistically significant compared to normal tissues. e Effect of V. amygdalina infusion on non-thiol proteins levels in glucose treated brain. Data = mean ± SD; n = 3. *Statistically significant compared to Glucose-only treated tissues; #Statistically significant compared to normal tissues
There was an increased NO level and myeloperoxidase activity on incubation of brain tissues with D-glucose only as shown in Fig. 3a, b, portraying an occurrence of proinflammation. Incubation with the infusion and metformin led to significant (p < 0.05) depleted level and activity.
Incubation of brain tissues with D-glucose only, caused a significant (p < 0.05) increase in ATPase activity as depicted in Fig. 4. This was significantly reduced dose – dependently on incubation with the infusion, with metformin showing little or no effect.
Molecular docking of d-glucose with catalase, myeloperoxidase, and ATPase revealed significant interactions, with ATPase showing the highest interaction (−5.45 kcal/mol) as depicted in Fig. 5a–c and Table 1. The core amino acid residues and hydrogen bond distance between the residues and ligand are shown in Table 1, with ATPase interaction having the highest number of residues (THRE, ASN, LEU and GLY) and atoms of residues (O, N and H).
Incubation of brain tissues with glucose only, led to the development of nitrate, amide II and amide I functional groups as shown in Fig. 6 and Table 2. Incubation with the infusion and metformin led to removal of these functional groups.
LC-MS characterization of V. amygdalina infusion revealed an alkaloid rich extract consisting of nicotinic acid, cumidine, and isoquinoline, 3-methyl- (Fig. 7). Amino acids consisting of l-serine, l-cysteine and l-proline were also identified. Other compounds identified were salicylic acid and γ-octalactone.
Analysis of the metabolites of the normal brain tissues revealed the presence of ganglioside, glucose, cardiolipin, triglyceride, inosine, molybdenum cofactor, monosaccharide, nucleotide, and iduronic acid metabolic intermediates as shown in Table 3. Incubation with d-glucose led to depletion of dTDP-D-glucose and ganglioside GM2 (d18:0/22:1(13Z)), with concomitant generation of 2-keto-glutaramic acid, cysteinyl-tyrosine, acetyl adenylate, CL(18:1(11Z)/18:1(11Z)/18:1(11Z)/18:1(11Z)), Ganglioside GM1 (d18:1/25:0), 4-Methylnonacosane, Ganglioside GT3 (d18:1/16:0) and Ganglioside GT3 (d18:0/16:0). Treatment with the infusion led regeneration of dTDP-D-glucose, with concomitant depletion of P1,P4-Bis(5′-uridyl) tetraphosphate, CL(18:2(9Z,12Z)/18:2(9Z,12Z)/18:2(9Z,12Z)/16:1(9Z)), 2-keto-glutaramic acid and cysteinyl-tyrosine. It also led to the generation of S-formylglutathione and adenosine tetraphosphate. Metformin led to regeneration of the glucose-induced depleted metabolites, with concomitant depletion of cysteinyl-tyrosine, acetyl adenylate, CL(18:1(11Z)/18:1(11Z)/18:1(11Z)/18:1(11Z)), ganglioside GT3 (d18:1/16:0) and ganglioside GT3 (d18:0/16:0), and generation of superoxide, glycerol 3-phosphate and guanosine tetraphosphate adenosine.
Pathway enrichment analysis of the identified metabolites revealed metabolic pathways for lactose synthesis, androstenedione, estrone, sucrose, amino sugar, androgen and estrogen, inositol, galactose, porphyrin, sphingolipid, and pyrimidine metabolisms in normal brain tissues (control) as shown in Table 4. These pathways were unaltered on incubation with d-glucose, with activation of pyruvate metabolism pathway. Although incubation with V. amygdalina infusion did not affect these pathways, the intensity fold of the pyruvate metabolism pathway was significantly lower than that of glucose-only treated tissues. Treatment with metformin deactivated the pyruvate metabolism pathway, with concomitant activation of de novo triacylglycerol biosynthesis, cardiolipin biosynthesis, degradation of superoxides, glycerol phosphate shuttle, mitochondrial electron transport chain, glycerolipid metabolism, and phospholipid biosynthesis pathways.
Except the amino acids, the other identified compounds of V. amygdalina infusion were predicted to be able to cross the blood brain barrier (Table 5). All the identified compounds were predicted to be orally safe as they fell between classes 4–6, except for l-serine which fell on class 2.
Discussion
Diminished brain glucose uptake has been recognized as one of the complication of T2D despite of chronic hyperglycemia (Vagelatos and Eslick 2013; Whitlow et al. 2015). This has been attributed to the down regulation of glucose transporters at the BBB, thus reducing facilitative transportation of glucose to the brain (Gejl et al. 2017; Hwang et al. 2017). Vernonia amygdalina amongst other medicinal plants have been reported for its antidiabetic and neuroprotective properties. To the best of our knowledge, for the first time, this study reports the ability of V. amygdalina to stimulate brain glucose uptake and its effect on brain metabolic pathways and metabolites.
The increased brain glucose uptake by the infusion (Fig. 1) indicates a glucose uptake facilitative potential of V. amygdalina. This can be attributed to the identified alkaloid, polyphenol and lactone constituents of the infusion (Fig. 7), as they were predicted to be BBB permeable (Table 5). Their predicted permeability across BBB may facilitate the transportation of glucose to the brain by the activation of glucose transporters notably GLUTs 1, 3 and 5 which are highly concentrated at the BBB (Simpson et al. 1994). Studies have reported the influence of phytochemicals from medicinal plants, particularly polyphenols, on the activation of glucose transporters (León et al. 2017; Williamson 2013). The reduced glucose uptake in brain tissues incubated with metformin can be attributed to the fact that metformin exhibits its action by the activation of GLUTs 2 and 4, which are less expressed at the BBB (Kellett and Brot-Laroche 2005; Rice et al. 2011). The increased glucose uptake by V. amygdalina can also be attributed to its ability to decrease ATPase activities in brain tissues (Fig. 4), as decreased ATPase activity, particularly the Na+/K+ ATPase have been implicated in facilitating the glucose transportation across the BBB (Falkowska et al. 2015; Magistretti and Allaman 2015). The high binding energy on docking D-glucose with ATPase (Fig. 5c and Table 1) portrays a strong molecular interaction, which is evident by the increased ATPase activity (Fig. 4) and decreased glucose uptake (Fig. 1) in brain tissues incubated with glucose only. Similarly, the increased ATPase activity in the metformin treated brain tissues (Fig. 4) corroborates its lower glucose uptake (Fig. 1).
Oxidative stress and inflammation have been reported for its influential role in the pathogenesis and progression of neuropathology (Das et al. 2009; Patel 2016), which have been attributed to the high consumption of O2 and glucose dependence by the brain (Patel 2016). Although the brain’s anatomy allows for a reductive environment which minimizes ROS generation, its low endogenous antioxidant system, redox-active metal load, polyunsaturated fatty acids, and excitotoxic and auto-oxidizable neurotransmitters dependence makes it prone to oxidative stress (Butterfield et al. 2001; Huang et al. 2004; Patel 2016). The depleted levels of GSH, NPT, and increased SOD and catalase activities, with concomitantly increased MDA level in brain tissues incubated with glucose only (Fig. 2a–e) depicts an occurrence of oxidative stress. This can be attributed to the activation of pyruvate metabolism pathway (Table 4). Activation of this pathway will lead to the production of lactate with concomitant generation of essential cofactor, NAD+. The continuous drive of this pathway lead to accumulation of lactate and increased generation of NAD+ which sustains the glycolytic flux, which in turn increases the turnover of glycolytic production of electron donor, NADH. This electron donor has been implicated in the production of ROS, as it inhibits the electron transport at complex II leading to the reduction of O2 to O2.- (Brownlee 2001; Du et al. 2001). The activation of this pathway can also be attributed to the decreased glucose uptake, owing to the need for the brain to switch energy source from glucose to ketones. This is evident by the presence of the ketone metabolite, 2-keto-glutaramic acid in the glucose-only treated tissue (Table 3). 2-keto-glutaramic acid can also act as a substrate for the enzyme, alanine transaminase which catalyzes the reversible conversion of alanine to pyruvate. The presence of sphingolipids and triglyceride derivatives (Table 3) in the glucose-only treated brain tissue, may be responsible for the increased MDA level (Fig. 2d) as they can act as substrates for lipid peroxidation. The strong molecular interactions between D-glucose and catalase (Fig. 5b and Table 1) suggests the potential of D-glucose to inhibit catalase activity.
The increased GSH and NTP levels, and SOD and catalase activities, with concomitant depletion of MDA level in the treated brain tissues indicate an antioxidative effect. The antioxidative effect of the infusion can be attributed to its identified phytochemical constituents particularly nicotinic acid, salicylic acid and γ-Octalactone, as these compounds have been reported for their antioxidant and neuroprotective activities (De La Cruz et al. 2004; Shoaib et al. 2017; Tupe et al. 2011). This can also be attributed to their predicted permeability across the BBB (Table 5), which corroborates other reports on the ability of polyphenols and alkaloids to cross the BBB (Youdim et al. 2003; Zhang et al. 2017). The standard antidiabetic drug, metformin caused an inhibition of the pyruvate metabolism, with concomitant activation of the glycerol phosphate shuttle and mitochondrial electron transport chain (Table 4). This indicates the activation of a proper channel of transporting electron donors generated by the glycolytic pathway, thus mopping up free radicals while generating ATPs for the brain use. This is corroborated by the superoxide and glycerol 3-phosphate metabolites (Table 3) and superoxide degradation pathway (Table 4), which is evident by its high SOD activity (Fig. 2b). The activated lipid metabolic pathways in the metformin treated tissues, indicates a maintenance of tissue integrity which may also be responsible for the decreased MDA level (Fig. 2d).
The increased NO level and myeloperoxidase activity (Fig. 3a, b) indicates an occurrence of proinflammation in the brain tissue incubated in glucose only. This is evident by the presence of nitrate functional group (Fig. 6 and Table 2). Similarly, the molecular interaction between D-glucose and myeloperoxidase (Fig. 5a and Table 1) indicates the potential of the former to activate the latter. The reversed level and activity on incubation with V. amygdalina infusion and metformin indicates an anti-proinflammatory activity, which is evident by the absence of the nitrate functional groups (Fig. 5a and Table 1). This corroborates previous reports on the anti-proinflammatory activity of V. amygdalina (Farombi and Owoeye 2011; Georgewill and Georgewill 2010), and can be attributed to the identified phytochemical constituents (Fig. 7) as well as their predicted BBB permeability (Table 5). Thus, further indicating the neuroprotective effect of V. amygdalina.
The predicted toxicity of the identified phytochemical constituents (Table 5) may indicate a relative safety of the infusion when ingested orally.
Conclusion
These results of this study suggest the ability of V. amygdalina to stimulate glucose uptake in brain tissue, with concomitant antioxidative and anti-proinflammatory activities. Thus, indicating its neuroprotective potential against diabetic brain. This can be attributed to the identified phytochemicals and their permeability across the BBB. Thus, further giving credence to the reports and folkloric use of this plant in the treatment of neurodegenerative diseases.
References
Adaramoye OA, Akintayo O, Achem J, Fafunso MA (2008) Lipid-lowering effects of methanolic extract of Vernonia amygdalina leaves in rats fed on high cholesterol diet. Vasc Heal Risk Manag 4:235–241
Adefegha SA, Rosa Leal DB, Olabiyi AA, Oboh G, Castilhos LG (2017) Hesperidin attenuates inflammation and oxidative damage in pleural exudates and liver of rat model of pleurisy. Redox Rep 22:563–571. https://doi.org/10.1080/13510002.2017.1344013
Adesanoye OA, Farombi EO (2010) Hepatoprotective effects of Vernonia amygdalina (astereaceae) in rats treated with carbon tetrachloride. Exp Toxicol Pathol 62:197–206
Adewoye O, Bolarinwa A, Olorunsogo O (2000) Ca++, Mg++-ATPase activity in insulin-dependent and non-insulin dependent diabetic Nigerians African. J Med Med Sci 29:195–199
Afolabi IS et al (2012) Solenostemon monostachyus, Ipomoea involucrata and Carica papaya seed oil versus glutathione, or Vernonia amygdalina: methanolic extracts of novel plants for the management of sickle cell anemia disease. BMC Complement Altern Med 12:262
Ajibola CF, Eleyinmi AF, Aluko RE (2011) Kinetics of the inhibition of renin and angiotensin I converting enzyme by polar and non-polar polyphenolic extracts of Vernonia amygdalina and Gongronema latifolium leaves. Plant Food Hum Nutr 66:320–327
Aslam M, Sial AA (2014) Neuroprotective effect of ethanol extract of leaves of Malva parviflora against amyloid-β-(Aβ-) mediated Alzheimer’s disease. Inter Scholar Res Not 2014:156976
Blair-Johnson M, Fiedler T, Fenna R (2001) Human myeloperoxidase: structure of a cyanide complex and its interaction with bromide and thiocyanate substrates at 1.9 Å resolution. Biochemistry 40:13990–13997
Brownlee M (2001) Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820
Butterfield DA, Drake J, Pocernich C, Castegna A (2001) Evidence of oxidative damage in Alzheimer's disease brain: central role for amyloid β-peptide. Trends Mol Med 7:548–554
Chan CX, Khan AA, Choi JH, Ng CD, Cadeiras M, Deng M, Ping P (2013) Technology platform development for targeted plasma metabolites in human heart failure. Clin Proteomics 10:7
Chance B, Maehly A (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Chikezie P (2006) Studies on the anti-sickling effects of Azadirachta indica and Vernonia amygdalina on HbSS erythrocytes. Inter J Nat Appl Sci 2:24–28
Chowdhury P, Soulsby M (2002) Lipid peroxidation in rat brain is increased by simulated weightlessness and decreased by a soy-protein diet. Ann Clin Lab Sci 32:188–192
Chukwuma CI, Islam MS (2015) Effects of xylitol on carbohydrate digesting enzymes activity, intestinal glucose absorption and muscle glucose uptake: a multi-mode study. Food Funct 6:955–962
Das S, Gautam N, Dey SK, Maiti T, Roy S (2009) Oxidative stress in the brain of nicotine-induced toxicity: protective role of Andrographis paniculata Nees and vitamin E. Appl Physiol Nutr Metab 34:124–135
De La Cruz J, Guerrero A, González-Correa J, Arrebola M, Sánchez De La Cuesta F (2004) Antioxidant effect of acetylsalicylic and salicylic acid in rat brain slices subjected to hypoxia. J Neurosci Res 75:280–290
Drwal MN, Banerjee P, Dunkel M, Wettig MR, Preissner R (2014) ProTox: a web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res 42:W53–W58
Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M (2001) Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest 108:1341–1348
Ebuehi OA, Ajagun-Ogunleye MO (2017) Neurochemical impact of the aqueous extract of Vernonia amygdalina and Talinum triangulare on learning and memory in male Wistar rats. Inter J Brain Cogn Sci 6:81–88
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Erukainure OL, Mopuri R, Oyebode OA, Koorbanally NA, Islam MS (2017a) Dacryodes edulis enhances antioxidant activities, suppresses DNA fragmentation in oxidative pancreatic and hepatic injuries; and inhibits carbohydrate digestive enzymes linked to type 2 diabetes. Biomed Pharmacother 96:37–47
Erukainure OL, Oyebode OA, Sokhela MK, Koorbanally NA, Islam MS (2017b) Caffeine–rich infusion from Cola nitida (kola nut) inhibits major carbohydrate catabolic enzymes; abates redox imbalance; and modulates oxidative dysregulated metabolic pathways and metabolites in Fe 2+−induced hepatic toxicity. Biomed Pharmacother 96:1065–1074
Erukainure OL, Hafizur RM, Kabir N, Choudhary MI, Atolani O, Banerjee P, Preissner R, Chukwuma CI, Muhammad A, Amonsou EO, Islam MS (2018) Suppressive effects of Clerodendrum volubile P Beauv.[Labiatae] Methanolic extract and its fractions on type 2 diabetes and its complications. Front Pharmacol 9:8. https://doi.org/10.3389/fphar.2018.00008
Falkowska A, Gutowska I, Goschorska M, Nowacki P, Chlubek D, Baranowska-Bosiacka I (2015) Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int J Mol Sci 16:25959–25981
Farombi EO, Owoeye O (2011) Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int J Environ Res Public Health 8:2533–2555
Frisch M et al (2009) Gaussian 09, revision D. 01. Gaussian, Inc., Wallingford CT,
Gejl M, Brock B, Egefjord L, Vang K, Rungby J, Gjedde A (2017) Blood-brain glucose transfer in Alzheimer’s disease: effect of GLP-1 analog treatment. Sci Rep 7:17490
Georgewill O, Georgewill U (2010) Evaluation of the anti-inflammatory activity of extract of Vernonia amygdalina. Asian Pac J Trop Med 3:150–151
Granell S, Gironella M, Bulbena O, Panés J, Mauri M, Sabater L, Aparisi L, Gelpí E, Closa D (2003) Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit Care Med 31:525–530
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
Howard C, Stevens J, Izevbigie E, Walker A, McDaniel O (2003) Time and dose-dependent modulation of phase 1 and phase 2 gene expression in response to treatment of MCF-7 cells with a natural anti-cancer agent. Cell Mol Biol (Noisy-le-Grand, France) 49:1057–1065
Huang X, Moir RD, Tanzi RE, Bush AI, Rogers JT (2004) Redox-active metals, oxidative stress, and Alzheimer's disease pathology. Ann N Y Acad Sci 1012:153–163
Hwang JJ, Jiang L, Hamza M, Sanchez Rangel E, Dai F, Belfort-DeAguiar R, Parikh L, Koo BB, Rothman DL, Mason G, Sherwin RS (2017) Blunted rise in brain glucose levels during hyperglycemia in adults with obesity and T2DM. JCI Insight 2
I.D.F (2018) IDF Diabetes Atlas. 8th edn. International Diabetes Federation
Ijeh II, Ejike CE (2011) Current perspectives on the medicinal potentials of Vernonia amygdalina Del. J Med Plant Res 5:1051–1061
Iwalokun B, Efedede B, Alabi-Sofunde J, Oduala T, Magbagbeola O, Akinwande A (2006) Hepatoprotective and antioxidant activities of Vernonia amygdalina on acetaminophen-induced hepatic damage in mice. J Med Food 9:524–530
Kakkar P, Das B, Viswanathan P (1984) A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys 21:130–132
Kellett GL, Brot-Laroche E (2005) Apical GLUT2: a major pathway of intestinal sugar absorption. Diabetes 54:3056–3062
Laursen M, Yatime L, Nissen P, Fedosova NU (2013) Crystal structure of the high-affinity Na+, K+-ATPase–ouabain complex with Mg2+ bound in the cation binding site. Proc Natl Acad Sci 110:10958–10963
León D, Uribe E, Zambrano A, Salas M (2017) Implications of resveratrol on glucose uptake and metabolism. Molecules 22:398
Magistretti PJ, Allaman I (2015) A cellular perspective on brain energy metabolism and functional imaging. Neuron 86:883–901
Maritim A, Sanders R, Watkins J III (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38
McEwen BS, Reagan LP (2004) Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur J Pharm 490:13–24
Mohebbatia R, Khazdairb MR, Hedayatia M (2017) Neuroprotective effects of medicinal plants and their constituents on different induced neurotoxicity. Method J Report Pharm Sci 6:34–50
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comp Chem 30:2785–2791
Ong KW, Hsu A, Song L, Huang D, Tan BKH (2011) Polyphenols-rich Vernonia amygdalina shows anti-diabetic effects in streptozotocin-induced diabetic rats. J Ethnopharmacol 133:598–607
Owoeye O, Farombi E, Onwuka S (2011) Gross morphometric reduction of rats’ cerebellum by gamma irradiation was mitigated by pretreatment with Vernonia amygdalina leaf extract. Romanian J Morphol Embryol 52:81–88
Pandit MK (2011) Neuroprotective properties of some Indian medicinal plants. Inter J Pharm Biol Arch 2
Pardridge WM, Triguero D, Farrell CR (1990) Downregulation of blood-brain barrier glucose transporter in experimental diabetes. Diabetes 39:1040–1044
Patel M (2016) Targeting oxidative stress in central nervous system disorders. Trends Pharmacol Sci 37:768–778
Pires DE, Blundell TL, Ascher DB (2015) pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J Med Chem 58:4066–4072
Reagan LP, Grillo CA, Piroli GG (2008) The As and Ds of stress: metabolic, morphological and behavioral consequences. Eur J Pharm 585:64–75
Rice S, Pellatt LJ, Bryan SJ, Whitehead SA, Mason HD (2011) Action of metformin on the insulin-signaling pathway and on glucose transport in human granulosa cells. J Clin Endocrinol Metab 96:E427–E435
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Saliu J, Ademiluyi A, Akinyemi A, Oboh G (2012) In vitro antidiabetes and antihypertension properties of phenolic extracts from bitter leaf (Vernonia amygdalina Del.). J Food Biochem 36:569–576
Sanner MF (1999) Python: a programming language for software integration and development. J Mol Graph Model 17:57–61
Saravanan S, Parimelazhagan T (2014) In vitro antioxidant, antimicrobial and anti-diabetic properties of polyphenols of Passiflora ligularis Juss. fruit pulp. Food Sci Human Wellness 3:56–64
Shoaib M, Shah I, Ali N, Adhikari A, Tahir MN, Shah SWA, Ishtiaq S, Khan J, Khan S, Umer MN (2017) Sesquiterpene lactone! A promising antioxidant, anticancer and moderate antinociceptive agent from Artemisia macrocephala jacquem. BMC Complement Altern Med 17:27
Simpson IA, Vannucci SJ, Maher F (1994) Glucose transporters in mammalian brain. Portland Press Limited
Tsikas D (2005) Review methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res 39:797–815
Tupe RS, Tupe SG, Agte VV (2011) Dietary nicotinic acid supplementation improves hepatic zinc uptake and offers hepatoprotection against oxidative damage. Brit J Nutr 105:1741–1749
Uddin R, Kim HH, Lee J-H, Park SU (2013) Neuroprotective effects of medicinal plants. EXCLI J 12:541
Vagelatos NT, Eslick GD (2013) Type 2 diabetes as a risk factor for Alzheimer's disease: the confounders, interactions, and neuropathology associated with this relationship. Epidemiol Rev 35:152–160
Whitlow C et al (2015) Effects of type 2 diabetes on brain structure and cognitive function: African American–diabetes heart study MIND. Amer J Neuroradiol 36:1648–1653
Williamson G (2013) Possible effects of dietary polyphenols on sugar absorption and digestion. Mol Nutr Food Res 57:48–57
Wishart DS et al (2012) HMDB 3.0—the human metabolome database in 2013. Nucl Acid Res 41: D801–D807
Wrighten SA, Piroli GG, Grillo CA, Reagan LP (2009) A look inside the diabetic brain: contributors to diabetes-induced brain aging. Biochim Biophys Acta (BBA) - Mol Basis Dis 1792:444–453
Xia J, Wishart DS (2016) Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Curr Prot Bioinform 14.10. 11–14.10. 91
Yang B, Hao F, Li J, Chen D, Liu R (2013) Binding of chrysoidine to catalase: spectroscopy, isothermal titration calorimetry and molecular docking studies. J Photochem Photobiol B Biol 128:35–42
Yeap SK, Ho WY, Beh BK, San Liang W, Ky H, Yousr AHN, Alitheen NB (2010) Vernonia amygdalina, an ethnoveterinary and ethnomedical used green vegetable with multiple bio-activities. J Med Plant Res 4:2787–2812
Yedjou C, Izevbigie E, Tchounwou PB (2008) Preclinical assessment of Vernonia amygdalina leaf extracts as DNA damaging anti-cancer agent in the management of breast cancer. Int J Environ Res Public Health 5:337–341
Youdim KA, Dobbie MS, Kuhnle G, Proteggente AR, Abbott NJ, Rice-Evans C (2003) Interaction between flavonoids and the blood–brain barrier: in vitro studies. J Neurochem 85:180–192
Zhang Y-N, Yang Y-F, Xu W, Yang X-W (2017) The blood-brain barrier permeability of six indole alkaloids from Uncariae Ramulus cum Uncis in the MDCK-pHaMDR cell monolayer model. Molecules 22:1944
Acknowledgements
This study was supported by a competitive research grant from the Research Office, University of KwaZulu-Natal (UKZN), Durban; an incentive grant for rated researchers and a grant support for women and young researchers from the National Research Foundation (NRF), Pretoria, South Africa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Erukainure, O.L., Oyebode, O.A., Ibeji, C.U. et al. Vernonia Amygdalina Del. stimulated glucose uptake in brain tissues enhances antioxidative activities; and modulates functional chemistry and dysregulated metabolic pathways. Metab Brain Dis 34, 721–732 (2019). https://doi.org/10.1007/s11011-018-0363-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-018-0363-7