Abstract
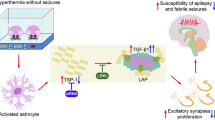
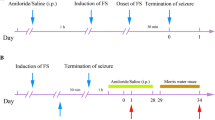
Levels of high mobility group box 1 (HMGB1), an important inflammatory mediator, are high in the serum of febrile seizure (FS) patients. However, its roles in FS and secondary epilepsy after prolonged FS are poorly understood. We demonstrate HMGB1’s role in the pathogenesis of hyperthermia-induced seizures (HS) and secondary epilepsy after prolonged hyperthermia-induced seizures (pHS). In the first experiment, 14–15-day-old male rats were divided into four groups: high-dose HMGB1 (100 μg), moderate-dose (10 μg), low-dose (1 μg), and control. Each rat was administered HMGB1 intranasally 1 h before inducing HS. Temperature was measured at seizure onset with electroencephalography (EEG). In the second experiment, 10–11-day-old rats were divided into four groups: pHS + HMGB1 (10 μg), pHS, HMGB1, and control. HMGB1 was administered 24 h after pHS. Video-EEGs were recorded for 24 h at 90 and 120 days old; histological analysis was performed at 150 days old. In the first experiment, the temperature at seizure onset was significantly lower in the high- and moderate-dose HMGB1 groups than in the control group. In the second experiment, the incidence of spontaneous epileptic seizure was significantly higher in the pHS + HMGB1 group than in the other groups. Comparison between pHS + HMGB1 groups with and without epilepsy revealed that epileptic rats had significantly enhanced astrocytosis in the hippocampus and corpus callosum. In developing rats, HMGB1 enhanced HS and secondary epilepsy after pHS. Our findings suggest that HMGB1 contributes to FS pathogenesis and plays an important role in the acquired epileptogenesis of secondary epilepsy associated with prolonged FS.






Similar content being viewed by others
References
Asano T, Ichiki K, Koizumi S et al (2011) High mobility group box 1 in cerebrospinal fluid from several neurological diseases at early time points. Int J Neurosci 121:480–484
Balosso S, Marosso M, Sanchez-Alaves M et al (2008) A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain 131:3256–3265
Baram TZ (1997) Febrile seizures: an appropriate-aged model suitable for long-term studies. Brain Res Dev Brain Res 98:265–270
Blümcke I, Pauli E, Clusmann H et al (2007) A new clinico-pathological classification system for mesial temporal sclerosis. Acta Neuropathol 113:235–244
Blümcke I, Thom M, Aronica E et al (2013) International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a task force report from the ILAE commission on diagnostic methods. Epilepsia 54:1315–1329
Camfield C, Camfield P (2005) Febrile seizures. In: Rojer EJ, Bureau M, Dravet P, Genton CA, Tassinari A, Wolf P (eds) Epileptic syndromes in infancy, childhood and adolescence, 4th edn. John Libbey Eurotext, Montrouge, pp 159–169
Cendes F, Kahane P, Brodie M, Andermann F (2012) The mesio-temporal lobe epilepsy syndrome. In: Bureau M, Genton P, Dravet C, Delgado-Escueta AV, Tassinari CA, Thomas P, Wolf P (eds) Epileptic syndrome in infancy, childhood, and adolescence, 5th edn. John Libbery Eurotext Ltd, Paris, pp 383–399
Choi J, Min HJ, Shin JE (2011) Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J Neuroinflammation 8:135
Choy M, Dubé CM, Patterson K et al (2014) A novel, noninvasive, predictive epilepsy biomarker with clinical potential. J Neurosci 34:8672–8684
Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA (2013) Glia and epilepsy: excitability and inflammation. Trends Neurosci 36:174–184
Dragnow M (1991) Adenosine and seizure termination. Ann Neurol 29:575
Dubé C, Vezzani A, Behrens M, Bartfai T, Baram TZ (2005) Interleukin-1beta contributes to the generation of experimental febrile seizures. Ann Neurol 57:152–155
Dubé C, Ravizza T, Hamamura M et al (2010) Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci 30:7484–7494
Frank MG, Weber MD, Watkins LR, Maier SF (2015) Stress sounds the alarmin: the role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun 48:1–7
Fukuda M, Morimoto T, Nagao H, Kida K (1997) Clinical study of epilepsy with severe febrile seizures and seizures induced by hot water bath. Brain and Development 19:212–216
Fukuda M, Morimoto T, Suzuki Y, Shinonaga C, Ishida Y (2007) Interleukin-6 attenuates hyperthermia-induced seizures in developing rats. Brain and Development 10:644–648
Fukuda M, Suzuki Y, Ishizaki Y et al (2009) Interleukin-1β enhances susceptibility to hyperthermia-induced seizures in developing rats. Seizure 18:211–214
Fukuda M, Suzuki Y, Hino H, Kuzume K, Morimoto T, Ishii E (2010) Adenosine A1 receptor blockage mediates theophylline-associated seizures. Epilepsia 51:483–487
Fukuda M, Hino H, Suzuki Y, Takahashi H, Morimoto T, Ishii E (2014) Postnatal interleukin-1β enhances adulthood seizure susceptibility and neuronal cell death after prolonged experimental febrile seizures in infantile rats. Acta Neurol Belg 114:179–185
Fukuda M, Ito M, Yano Y et al (2015) Postnatal interleukin-1β administration after experimental prolonged febrile seizures enhances epileptogenesis in adulthood. Metab Brain Dis 30:813–819
Hino H, Takahashi H, Suzuki Y, Tanaka T, Ishii E, Fukuda M (2012) Anticonvulsive effect of paeoniflorin on experimental febrile seizures in immature rats: possible application for febrile seizures in children. PLoS One 7:e42920
Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S (1996) A novel gene Iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun 224:855–862
Kanemoto K, Kawasaki J, Yuasa S et al (2003) Increased frequency of interleukin-1beta-511T allele in patients with temporal lobe epilepsy, hippocampal sclerosis, and prolonged febrile convulsion. Epilepsia 44:796–799
Kim JB, Sig Choi J, YM Y et al (2006) HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci 26:6413–6421
Kira R, Torisu H, Takemoto M et al (2005) Genetic susceptibility to simple febrile seizures: interleukin-1beta promoter polymorphisms are associated with sporadic cases. Neurosci Lett 26:239–244
Kleen JK, Holmes GL (2010) Taming TLR4 may ease seizures. Nat Med 16:369–370
Lewis DV, Shinnar S, Hesdorffer DC et al (2014) Hippocampal sclerosis after febrile status epilepticus: the FEBSTAT study. Ann Neurol 75:178–185
Maroso M, Balosso S, Ravizza T et al (2010) Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16:413–420
Morimoto T, Nagao H, Sano N, Takahashi M, Matsuda H (1991) Electroencephalographic study of rat hyperthermic seizures. Epilepsia 32:289–293
Müller S, Ronfani L, Bianchi ME (2004) Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med 255:332–343
Omran A, Peng J, Zhang C et al (2012) Interleukin-1β and microRNA-146a in an immature rat model and children with mesial temporal lobe epilepsy. Epilepsia 53:1215–1224
Pitkänen A, Sutula TP (2002) Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol 1:173–181
Proper EA, Hoogland G, Kappen SM et al (2002) Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 125:32–43
Racine RJ (1972) Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol 32:281–294
Ravizza T, Noé F, Zardoni D, Vaghi V, Sifringer M, Vezzani A (2008) Interleukin converting enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1β production. Neurobiol Dis 31:327–333
Rogawski MA, Löscher W (2004) The neurobiology of antiepileptic drugs. Nat Rev Neurosci 5:553–564
Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW (2007) Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol 35:984–999
Tanabe T, Hara K, Shimakawa S, Fukui M, Tamai H (2011) Hippocampal damage after prolonged febrile seizure: one case in a consecutive prospective series. Epilepsia 52:837–840
Viviani B, Bartesaghi S, Gardoni F et al (2003) Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 24:8692–8700
Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF (2015) Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J Neurosci 35:316–324
Youn JH, YJ O, Kim ES, Choi JE, Shin JS (2008) High mobility group box 1 protein binding to lipopolysaccharide facilitates transfer of lipopolysaccharide to CD14 and enhances lipopolysaccharide-mediated TNF-α production in human monocytes. J Immunol 180:5067–5074
Acknowledgements
We are grateful to the staff of the Animal Center of ADRES of Ehime University for their gentle care of our animals and histological staining. This study was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 25461553 & 16 K09990).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All experimental procedures conformed to the guidelines from the Ministry of Education of Japan and were approved by the animal experimental committee of Ehime University Graduate School of Medicine (No. TE-17-2).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ito, M., Takahashi, H., Yano, H. et al. High mobility group box 1 enhances hyperthermia-induced seizures and secondary epilepsy associated with prolonged hyperthermia-induced seizures in developing rats. Metab Brain Dis 32, 2095–2104 (2017). https://doi.org/10.1007/s11011-017-0103-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-017-0103-4