Abstract
Approximately 25 % of clinically important drugs and numerous environmental carcinogens are metabolised by CYP2D6. Variation in the CYP2D6 gene and concomitant use of tamoxifen (TAM) with certain antidepressants may increase recurrence risk in breast cancer patients due to reduced enzyme activity. In this study we determined the appropriateness of adding CYP2D6 genotyping to the breast cancer genetic testing options already available in South Africa, which include BRCA mutation screening and transcriptional profiling to assess estrogen receptor (ER) status. A total of 114 South African breast cancer patients, including 52 Caucasian and 62 Coloured (Mixed ancestry), and 63 Caucasian control individuals were genotyped for the most common inactivating allele (CYP2D6*4, rs3892097) previously identified in the CYP2D6 gene. In the initial validation data set consisting of 25 Caucasian and 62 Coloured patients, the CYP2D6*4 allele frequency was significantly higher in Caucasian compared to Coloured patients (24 % vs. 3 %, p < 0.001), similar to previous findings in the general South African population. Extended CYP2D6 genotyping was subsequently performed in an implementation data set of 27 Caucasian breast cancer patients, to determine the prevalence of depression and use of antidepressants in a clinical setting. A medical history of depression and/or use of antidepressants was reported in 37 % (10/27) of these breast cancer patients genotyped for CYP2D6*4. This translational research study has led to increased awareness among clinicians of the potential benefits of CYP2D6 genotyping to facilitate prevention of cumulative risk in a high-risk genetic subgroup of breast cancer patients considered for concomitant treatment of TAM and antidepressants that may reduce enzyme function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Depression is the most common psychiatric problem in cancer patients. As the age at diagnosis of breast cancer decreases in the general population, the risk of depression becomes higher (Kim et al. 2010). Younger patients suffer more from psychiatric distress, resulting in negative effects on quality of life and survival rate. The treatment of depression as part of a comprehensive risk reduction approach is therefore an important consideration in patients with breast cancer. It has been reported that between 20 % and 30 % of breast cancer patients treated with Tamoxifen (TAM) to reduce recurrence risk, also use antidepressants for depression or hot flashes (Nelson et al. 2006; Kim et al. 2010).
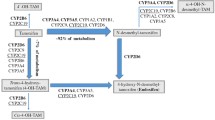
TAM is the most commonly used anti-estrogen drug for treatment of breast cancer, due to its active metabolites, hydroxytamoxifen and endoxifen. Selective serotonin reuptake inhibitor (SSRI) antidepressants can decrease the effectiveness of TAM because these drugs compete for the cytochrome P450 2D6 (CYP2D6) enzyme that metabolises TAM (Jin et al. 2005; Bernard et al. 2006). Consequently, antidepressants that act as CYP2D6 inhibitors may increase the risk of breast cancer relapse (Goetz et al. 2007). Antidepressants such as paroxetine, fluoxetine, and bupropion strongly inhibit CYP2D6 enzyme activity, whereas sertraline, duloxetine and diphenhydramine are considered moderate inhibitors. Escitalopram (Lexapro, Cipralex) venlafaxine (Effexor) and citalopram appear to have a very mild or no inhibiting effect on CYP2D6 activity (Preskorn et al. 2007; Lash et al. 2008; Holzman 2009).
Reduction in CYP2D6 activity due to enzyme inhibitors or polymorphisms in the CYP2D6 gene has been shown to reduce endoxifen levels (Borges et al. 2006) and may be associated with poorer outcome in women with breast cancer who have been treated with TAM (Jin et al. 2005; Goetz et al. 2007; Newman et al. 2008). In addition to antidepressants and TAM, CYP2D6 also metabolises antipsychotics, antiarrhythmic agents (propafenone, flecainide), beta-blockers (timolol, metoprolol, alprenolol), and opioids such as codeine and dextromethorphan. Due to CYP2D6 expression in the brain this enzyme furthermore mediates neuroprotection through inactivation of neurotoxins and breakdown of endogenous neural compounds such as catecholamines (Mann et al. 2011). Poor metabolisers have two copies of a defective CYP2D6 gene, and as a result they metabolise drugs at a much slower rate. This may result in a greater potential for adverse events and lower efficacy of drugs requiring CYP2D6 activation (Bernard et al. 2006).
Strong evidence has been provided that treatment failure due to impaired drug metabolism may increase the risk of recurrence of many different cancer types, not only in sporadic cases but also in patients with familial breast cancer (Newman et al. 2008). Various genes have been implicated in the development of breast cancer and those most studied include the BRCA1 and BRCA2 genes. Germline mutations in these two highly penetrant susceptibility genes explain the majority of familial breast cancer and additionally increase the risk for developing ovarian cancer (Sluiter and van Rensburg 2011). The lifetime risk for developing breast cancer is 65 % to 85 % for BRCA1 and 45 % to 85 % for BRCA2 mutation carriers (Chen and Parmigiani 2007). Increased frequencies of specific BRCA1 and BRCA2 mutations have been described in the South African Caucasian (Afrikaans-speaking) and Coloured (Mixed ancestry) populations (Reeves et al. 2004; Agenbag 2005; van der Merwe et al. 2012). Three founder mutations [(BRCA1 c.1374delC (1493delC), BRCA1 c.2641G>T (2760 G>T, E881X) and BRCA2 c.7934delG (8162delG)] account for approximately 90 % of all BRCA mutation-positive families in the Afrikaner population of European descent (van der Merwe and van Rensburg 2009). A relatively frequent BRCA2 founder mutation c.5771_5774delTTCA (5999del4) has also recently been identified in 3.4 % of Coloured and 25 % of Black Xhosa breast cancer patients studied in the Western Cape region of South Africa (van der Merwe et al. 2012).
The vast majority of breast cancers in postmenopausal women are estrogen-receptor (ER)-positive (Punglia et al. 2008). However, in premenopausal breast cancer patients who are more likely to be carriers of BRCA1 or BRCA2 familial mutations, tumours display differences with regard to hormone receptors. While BRCA1 tumours are frequently ER and progesterone receptor negative, BRCA2 tumours are more commonly positive for both receptors (Lomen et al. 1998). The lack of hormone receptor positivity in most BRCA1 tumours suggests that treatment with TAM or other hormonal therapy may be less effective in this patient population. In a study performed by Newman et al. (2008), in which TAM-treated familial breast cancer patients were investigated for the effect of reduced CYP2D6 activity on clinical outcome, it was shown that the poor metaboliser status predicted worse overall survival in patients with familial breast cancer. Contrary to several other studies, the effect on enzyme function caused by both genetic variation in the CYP2D6 gene and concomitant use of a potent CYP2D6 inhibitor was taken into account. Patients with BRCA2 mutations had a significantly worse overall survival compared to patients with BRCA1 tumours (median survival: 7 versus 28 years; P = 0.008; adjusted hazard ratio, 9.7). Based on the evidence provided in this study, it is of utmost importance to identify genetic subgroups of breast cancer patients most likely to benefit from CYP2D6 genotyping. Similar to BRCA mutation testing performed only in selected patients who fulfil the test criteria, CYP2D6 genotyping may only be applicable in a subgroup of breast cancer patients (e.g. BRCA2- and ER-positive patients considering concomitant use of TAM and antidepressants).
In light of the increased frequency of particularly BRCA2 mutations in three different South African population groups and the fact that use of certain antidepressants can significantly reduce the function of the CYP2D6 gene, implementation of a pharmacogenetics CYP2D6 assay is an important consideration. The aim of this study was therefore twofold: 1) to determine the frequency of the relatively common inactivating CYP2D6*4 allele in South African breast cancer patients (validation data set) and 2) to determine the prevalence of depression/use of antidepressants in breast cancer patients referred for genetic testing (implementation data set). Ultimately, breast cancer patients may benefit from the implementation of pathology supported genetic testing (PSGT) that combines relevant clinical and laboratory information for clinical decision-making. Although evidence-based guidelines should ideally be developed before implementation of genomic applications, Khoury et al. (2007) acknowledged the need to fill the remaining information gaps through ongoing data collection and health outcome studies. PSGT involves a combined service and research approach that seeks to evaluate “real world” health outcomes of genomic applications (Kotze and van Rensburg 2012), whereby overlapping aspects of translation research could provide feedback loops to allow integration of new genetic knowledge into clinical care (Khoury et al. 2007).
Subjects and methods
This study was approved by the Ethics Research Committee of the University of Stellenbosch and was therefore performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Study participants
This investigation consisted of a validation and implementation phase. The validation data set included 87 unrelated female breast cancer patients between the ages of 30 and 87 years, previously subjected to BRCA1 and BRCA2 mutation analysis (Agenbag 2005, MJ Kotze, unpublished data). DNA samples of 63 unrelated Caucasian individuals (42 women and 21 men) were available as controls for inclusion in the analytical validation phase of the project. The implementation data set included 27 Caucasian breast patients recently referred for genetic testing by private practicing clinicians; data was made available for this component of the study from an online genetic database (http://www.gknowmix.org). Written informed consent was obtained from all study participants. A questionnaire was used to document clinical characteristics and additional information was obtained from patient records.
Analytical assay validation and CYP2D6 genotyping
Conventional polymerase chain reaction (PCR) followed by direct DNA sequencing was performed for detection of the inactivating CYP2D6*4 allele (rs3892097, also known as c.1934G>A or 1846G>A) (http://genetmed.fudan.edu.cn/dbHCCvar/result.asp), in 5 internal control DNA samples (K1-5) obtained from healthy individuals. Direct sequencing of the PCR fragments resulted in the identification of the wild type, heterozygous and homozygous genotypes related to the CYP2D6*4 allele. DNA samples of 63 unrelated Caucasian individuals (42 women and 21 men) were subsequently used for analytical validation of high throughput CYP2D6 genotyping against direct DNA sequencing as the gold standard. DNA extracted from 114 breast cancer patients (87 from the validation set and 27 from the implementation set) were subjected to CYP2D6 genotyping using the ABI™ TaqMan® SNP Genotyping assay on the ABI 7900HT real-time apparatus.
Only the CYP2D6*4 allele was analysed in the study population to evaluate the appropriateness of CYP2D6 testing in clinical practice, since it was shown to be the most common inactivating single nucleotide polymorphism (SNP) in the CYP2D6 gene. Based on the outcome of the study, a more comprehensive pharmacogenetic assay may be developed that will include the six inactivating alleles (*3, *4, *5, *6, 7 and *8) found to be responsible for 95 % of the poor metaboliser status (Arneth et al. 2009).
Statistical analysis
Allele frequencies and genotype distribution were determined by gene counting and Hardy Weinberg equilibrium was tested using an exact test. Linear models were used to test for an association between age of breast cancer diagnosis and presence of a family history of cancer. This analysis was done in all patients while adjusting for previous personal medical history of cancer, ethnic group, body mass index, alcohol intake, smoking and CYP2D6 genotype as fixed effects. All analyses were done using the R software environment and the R genetics package, both of which are freely available from http://www.r-project.org. Results corresponding to p-values below 0.05 are described as significant.
Results
The clinical characteristics of the 87 breast cancer patients previously screened for mutations in the BRCA1 and 2 genes are summarised in Table 1. The majority of patients were postmenopausal. Approximately half of these patients in the validation data set had a family history of cancer, with early onset (<50 years) breast cancer reported in 26 % of patients. The age at diagnosis of breast cancer in the study population was significantly associated with a family history of cancer (p < 0.01), independent of various possible confounders including ethnicity, body mass index, alcohol intake, smoking or CYP2D6*4 genotype (data not shown). A family history of cancer, expected to be closely related to BRCA mutation status, reduced the expected average age at diagnosis/onset of breast cancer with 8 years. A similar effect on age of breast cancer diagnosis was not observed in relation to the presence or absence of the CYP2D6*4 allele.
The allelic distribution of CYP2D6*4 was found to be in Hardy Weinberg Equilibrium in both the breast cancer patients and the controls. Genotype distribution and allele frequencies differed significantly between the Caucasian and Coloured population groups (p < 0.001). The frequency of the CYP2D6*4 allele and the poor metaboliser status characterised by the homozygous genotype were highest in the Caucasian breast cancer patients. No significant differences in genotype distribution or allele frequencies were observed between the Caucasian patient and control groups (Table 2). The 21 male controls were excluded from this comparative analysis performed only in female breast cancer patients.
As indicated in Table 3, two of the six (33 %) BRCA-positive patients included in the study were homozygous for the inactivating CYP2D6*4 allele. Both poor metabolisers (Caucasian) were ER-positive; one patient tested positive for a mutation in the BRCA1 gene and the other was BRCA2-positive. The BRCA2 mutation-positive patient (sample 22) provided an example of how CYP2D6 genotyping may affect clinical management. Instead of TAM, an alternative endocrine treatment was administered after the patient underwent a hysterectomy due to her risk profile. Direct DNA sequencing confirmed the real-time PCR result of CYP2D6*4 homozygosity as obtained with the TaqMan genotyping assay using the ABI 7900HT, as well as the Corbett Rotor-Gene RT-PCR system (data not shown).
The above-mentioned findings prompted extended CYP2D6 mutation analysis in an implementation data set of 27 breast cancer patients, to also assess the use of antidepressants in TAM-treated breast cancer patients or patients who considered TAM treatment. Ten patients (37 %) referred by participating clinicians reported a medical history of depression, with current use of antidepressants reported in four patients (15 %). None of these patients were homozygous for CYP2D6*4, while 4 heterozygotes were identified (Table 4).
Knowledge of the different classes of CYP2D6 inhibitors is very important in the heterozygous patients as their ability to effectively metabolise TAM could be affected by reduced enzyme activity caused by both the intermediate metaboliser status due to CYP2D6*4 heterozygosity and concomitant use of antidepressants. For example, one CYP2D6*4 heterozygous breast cancer patient (sample J) was diagnosed with depression at the age of 48 years and had been on various medications, including Wellbutrin (bupropion) known to strongly inhibit CYP2D6 function (Table 4). Drug side effects with use of antidepressants and various other medications were also reported. This ER-positive overweight patient with high cholesterol levels had bilateral breast cancer with 4 recurrent events between the ages of 39 and 57 years. She tested negative for three founder mutations in the BRCA1 and BRCA2 genes.
Discussion
Identification of genetic subgroups of the population that are at increased risk of cancer initiation, progression or recurrence due to heritable susceptibility or ineffective therapeutic approaches is an important clinical consideration. Currently, DNA-based BRCA 1 and 2 gene testing and RNA-based transcriptional profiling including the determination of ER status are offered as a routine service to South African patients who fulfil the criteria for these tests (Kotze et al. 2005). This study aimed to determine the appropriateness of adding CYP2D6 genotyping to the breast cancer genetic test panel. The first step was to determine the frequency of the most common inactivating CYP2D6*4 allele in South African breast cancer patients (validation data set). The clinical usefulness of providing information related to CYP2D6 genotype to participating clinicians was furthermore assessed in a pilot study focused on the prevalence of depression and use of antidepressants in breast cancer patients subjected to diagnostic genetic testing (implementation data set). Since reduced CYP2D6 activity can be caused by variation in the CYP2D6 gene and/or the use of certain antidepressants, both aspects were considered in this study.
Breast cancer is most prevalent amongst South African Caucasian and Asian women and is the second most common cancer among Black and Coloured women (Vorobiof et al. 2001). This observation raises the question of whether genetic risk factors may explain ethnic differences in breast cancer risk. In this study, a significantly higher frequency of the CYP2D6*4 allele was detected in Caucasian (24 %) compared with Coloured breast cancer patients (3 %). This finding is however unlikely to account for the higher prevalence of breast cancer in the Caucasian compared to Coloured group, since CYP2D6*4 affects drug response and has not been associated with an increased risk of primary breast cancer (Goetz et al. 2007). This is in accordance with the failure to detect a difference in allele frequencies for CYP2D6*4 between the Caucasian patient and control groups. Since CYP2D6*5 is relatively common in the Coloured population (Gaedigk and Coetsee 2008), future mutation analysis should be extended to include this assay. More than 75 CYP2D6 allelic variants have been identified to date (Bernard et al. 2006) and six inactivating alleles (*3, *4, *5, *6, *7 and *8) are responsible for approximately 95 % of the poor metaboliser status in most populations (Arneth et al. 2009).
The current debate in the literature as to whether CYP2D6 pharmacogenetic testing of breast cancer patients has clinical relevance highlights the importance of defining the selection criteria for a subgroup of patients where the potential benefits would outweigh the risk of unnecessary genetic testing. No statistically significant associations were observed between CYP2D6 genotype and recurrence risk in TAM-treated patients in recent clinical trials (Rae et al. 2012; Regan et al. 2012). In contrast, strong evidence provided by numerous previous studies showed that CYP2D6 genotype is directly (Newman et al. 2008) as well as indirectly (Jin et al. 2005) associated with the survival of breast cancer patients treated with TAM (Kiyotani et al. 2010). In a study by Schroth et al. (2009) performed in 1,325 patients, recurrence rates were 14.9 % for extensive (wild-type genotype), 20.9 % for intermediate (heterozygotes) and 29 % for poor metabolisers (homozygotes) at 9 years of follow-up. All-cause mortality rates were 16.7 %, 18.0 % and 22.8 %, respectively. Conflicting results reported in the literature may be attributed to differences related to TAM combination therapy, genotyping comprehensiveness, and CYP2D6 inhibitor coadministration (Hertz et al. 2012). These include frequent use of antidepressants prescribed to treat depression in breast cancer patients and/or menopausal vasomotor symptoms such as hot flashes. TAM treatment may induce or aggravate depression or anxiety, and commonly causes hot flashes (Kim et al. 2010). During the research-focused implementation phase of our study, it was found that 37 % (10/27) of breast cancer patients from the implementation data set suffers from depression, while the use of antidepressants for depression were documented in four patients (15 %). These findings are in agreement with previous studies which reported that between 20 % and 30 % of TAM-treated breast cancer patients also use antidepressants (Nelson et al. 2006; Kim et al. 2010).
TAM is the standard 5 year endocrine treatment for premenopausal and postmenopausal women with ER-positive breast cancer. In contrast, aromatase inhibitors are frequently prescribed for postmenopausal women (Kim et al. 2010). The use of aromatase inhibitors has been prohibited in premenopausal breast cancer patients due to the activation of ovarian functions implicated in polycystic ovarian diseases. In premenopausal patients for whom the use of aromatase inhibitors is inappropriate, TAM is the treatment of choice. Given the link between CYP2D6 genotype status and clinical outcome in risk allele carriers on TAM, it is likely that aromatase inhibitors would be the preferred treatment for postmenopausal women with deficiencies in TAM metabolism (Kim et al. 2010; Punglia et al. 2008). Newman et al. (2008) suggested that for postmenopausal patients with BRCA2 mutations and the CYP2D6 poor metaboliser genotype, an aromatase inhibitor would be the drug of choice.
The above-mentioned recommendation was of particular relevance in at least one patient with a founder BRCA2 mutation found to be homozygous for the CYP2D6*4 allele (sample 22). This ER-positive breast cancer patient reported a strong family history of breast cancer, which was shown in this study to be associated with an 8 year (on average) earlier onset of breast cancer irrespective of whether a mutation has been identified in the BRCA1 or BRCA2 genes. Based on this patient’s CYP2D6*4 genotype and the fact that she had a hysterectomy, the treating oncologist prescribed an aromatase inhibitor instead of TAM treatment. The proven clinical utility of CYP2D6 genotyping resulting in a change in clinical management in this patient, has led to increased awareness of the potential consequences of CYP2D6 inactivation. This has also translated into avoidance of concomitant use of TAM and inactivating antidepressants in an increasing number of breast cancer patients. The CYP2D6*4 heterozygote who had at least four episodes of recurrent breast cancer between the ages of 39 and 57 years (sample J) provides an example of where this information could have been relevant if provided when she first presented with bilateral breast cancer. She has been diagnosed with depression at the age of 48 years and has subsequently been treated with three different antidepressants, of which the use of Wellbutrin (bupropion) reported at entry into this study is known to strongly inhibit CYP2D6 activity. This ER-positive breast cancer patient reported side effects with use of antidepressants and various other medications. Arimidex (anastrozole), an aromatase inhibitor, was subsequently prescribed due to her current postmenopausal status. This case provides insight of how a patient’s quality of life and well-being could be adversely affected by reduced CYP2D6 activity compromised further by use of antidepressants metabolised by CYP2D6. Extended mutation analysis of the CYP2D6 gene as well as the entire BRCA1 and 2 genes are considered in this patient to further investigate the cause of her poor treatment outcome.
The above-mentioned information explains what is meant by Khoury et al. (2007), who stated that evaluation of the interaction between providers and patients would enable the assessment of risks and benefits outside the context of randomized clinical trials. These authors acknowledged that while the translation research pathway for therapeutics is relatively straightforward, it is less clear for genetic tests that may provide useful information only in a subgroup of patients in some populations. Lorizio et al. (2011) found that CYP2D6 genotyping in breast cancer patients affects choice of therapy even in the absence of definitive data on clinical impact. This confirms the value of genetic information to guide treatment decisions on an individual basis, compared to a one-size-fits-all approach applied in most clinical trials. CYP2D6 genotyping is particularly relevant in the local population due to the increased frequency of BRCA2 founder mutations (van der Merwe et al. 2012) and the high recurrence risk associated with reduced CYP2D6 activity in mutation-positive patients (Newman et al. 2008). Since CYP2D6*4 is virtually absent in the Black South African population (Wright et al. 2010), genotyping for this allele would not be appropriate in this particular ethnic group.
We believe that the same path used for development and implementation of genetic tests for high-penetrance genetic variations identified in the BRCA1 and 2 genes should be followed for low-penetrance genetic variations such as those in the CYP2D6 gene, with the exception that known environmental exposures must also be taken into account. Integration of CYP2D6 genotype information into the clinical decision-making process would require careful consideration of all possible determinants of CYP2D6 metabolic activity. Although all determinants of CYP2D6 metabolic activity have not yet been identified and some are poorly understood, current knowledge related to the consequences of concomitant use of TAM and CYP2D6 inhibitors is well-documented and needs to be communicated to clinicians and their patients. The phenotypic expression of genetic variation in the CYP2D6 gene is modulated by various external factors including physiological states (e.g. pregnancy) and certain herbal products. Borges et al. (2010) used genotype, concomitant medication and phenotype data to develop a CYP2D6 activity score that incorporates both CYP2D6 genotype and CYP2D6-mediated drug interactions. Correlation of this score with CYP2D6 phenotype represented a significant improvement over the use of CYP2D6 genotype alone.
Multiple gene/mutation testing in the context of the patient’s medical and/or family history provides a basis for development and formulation of personalised cancer prognostication, drug treatment and lifestyle-related risk reduction programs (Cleator and Ashworth 2004). CYP2D6 genotyping has recently been included as the pharmacogenetics component of a multi-gene test for cardiovascular disease (CVD) (Kotze et al. 2003; Kotze and van Rensburg 2012), due to increased risk of muscle pain in patients taking cholesterol-lowering medication metabolised by CYP2D6 (Frudakis et al. 2007). Due to the potential significance of CYP2D6 genotyping in breast cancer patients and the increased risk of thrombophilia and cardiac complications with the use of chemotherapy and hormonal treatment (e.g. TAM, HRT), the development and validation of a comprehensive screening strategy is hereby warranted to improve well-being and quality of life. In this study the PSGT approach—initially developed for risk management of CVD and related medical conditions (Kotze and van Rensburg 2012)—was applied during the implementation phase of this study to assess the clinical usefulness of combining diagnostic and pharmacogenetic testing in breast cancer patients. Assessment of CVD risk factors in breast cancer patients is supported by the direct link between an increased risk of postmenopausal breast cancer and the metabolic syndrome characterised by central obesity, insulin resistance, hypertension and/or hyperlipidemia (high triglycerides and/or low HDL-cholesterol levels) (Rosato et al. 2011). These findings support the implementation of CYP2D6 genotyping as part of a multi-gene test that takes co-morbidities such as obesity (King et al. 2003; Li et al. 2009), family history, ethnicity, ER status, BRCA mutation status and use of antidepressants into account prior to TAM treatment of breast cancer patients. Although CYP2D6*4 is not deterministic by itself to cause disease, it may become clinically relevant in the presence of environmental co-factors or co-inheritance with other genetic risk factors (Mellick 2006; Newman et al. 2008).
This study contributed to an increased awareness of the important role clinicians can play in identifying breast cancer patients who may benefit from CYP2D6 genotyping, based on their unique genetic background and concomitant use of TAM and certain antidepressants. While it remains uncertain whether a pharmacogenetic profile should be obtained prior to initiating TAM treatment, we conclude that genetic testing performed as part of a comprehensive risk reduction strategy would provide useful support for clinical decision-making in selected patients. Two of the six (33 %) BRCA-positive patients included in the validation data set were homozygous for the inactivating CYP2D6*4 allele. Prospective studies are therefore warranted to determine the effect of CYP2D6 status on health outcomes in BRCA-positive South African breast cancer patients treated with TAM.
CYP2D6 genotyping has relevance in several clinical domains and represents one of several pieces of information that clinicians may consider in guiding the choice of therapy for each patient. This includes an important role in cardiovascular pharmacogenomics that may be relevant in some breast cancer patients. The weight of current scientific evidence in relation to risk-benefit assessment supports CYP2D6 genotyping in breast cancer patients who (1) are receiving TAM and (2) are at high risk for tumour recurrence (e.g. family history, BRCA1/2 mutation-positive) or (3) are required to take potential competing antidepressants.
References
Agenbag GM (2005) Molecular genetic analysis of familial breast cancer in South Africa. Dissertation, Stellenbosch University. http://hdl.handle.net/10019/953
Arneth B, Shams M, Hiemke C, Hartter S (2009) Rapid and reliable genotyping procedure for detection of alleles with mutations, deletion, or/and duplication of the CYP2D6 gene. Clin Biochem 42:1282–1290
Bernard S, Neville KA, Nguyen AT, Flockhart DA (2006) Interethnic differences in genetic polymorphisms of CYP2D6 in the U.S. population: clinical implications. Oncologist 11:126–135
Borges S, Desta Z, Li L et al (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 80:61–74
Borges S, Desta Z, Jin Y et al (2010) Composite functional genetic and comedication CYP2D6 activity score in predicting tamoxifen drug exposure among breast cancer patients. J Clin Pharmacol 50:450–458
Chen S, Parmigiani G (2007) Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol 25:1329–1333
Cleator S, Ashworth A (2004) Molecular profiling of breast cancer: clinical implications. Br J Cancer 90:1120–1124
Frudakis T, Thomas M, Ginjupalli S et al (2007) CYP2D6*4 polymorphism is associated with statin-induced muscle effects. Pharmacogenet Genomics 17:695–707
Gaedigk A, Coetsee C (2008) The CYP2D6 gene locus in South African coloureds: unique allele distributions, novel alleles and gene arrangements. Eur J Clin Pharmacol 64:465–475
Goetz MP, Knox SK, Suman VJ et al (2007) The impact of cytochrome P450 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res Treat 101:113–121
Hertz DL, McLeod HL, Irvin WJ Jr (2012) Tamoxifen and CYP2D6: A Contradiction of Data. Oncologist. Apr 24. [Epub ahead of print]
Holzman D (2009) Tamoxifen, antidepressants, and CYP2D6: the conundrum continues. J Natl Cancer Inst 101:1370–1371
Jin Y, Desta Z, Stearns V et al (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30–39
Khoury MJ, Gwinn M, Yoon PW et al (2007) The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genet Med 9:665–674
Kim S, Lee M, Lee K et al (2010) Use of antidepressants in patients with breast cancer taking tamoxifen. J Breast Cancer 13:325–336
King MC, Marks JH, Mandell JB, the New York Breast Cancer Study Group (2003) Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302:643–646
Kiyotani K, Mushiroda T, Imamura CK et al (2010) Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol 28:1287–1293
Kotze MJ, van Rensburg SJ (2012) Pathology supported genetic testing and treatment of cardiovascular disease in middle age for prevention of Alzheimer’s disease. Metab Brain Dis. doi:10.1007/s11011-012-9296-8
Kotze MJ, Kriegshäuser G, Thiart R et al (2003) Simultaneous detection of multiple familial hypercholesterolaemia mutations facilitates an improved diagnostic service in South African patients at high risk of cardiovascular disease. Mol Diagn 7:169–174
Kotze MJ, Malan J, Pienaar R, Apffelstaedt J (2005) The role of molecular genetic testing in modern breast health management. S Afr Fam Pract 47:38–40
Lash TL, Pedersen L, Cronin-Fenton D (2008) Tamoxifen’s protection against breast cancer recurrence is not reduced by concurrent use of the SSRI citalopram. Br J Cancer 99:616–621
Li CI, Daling JR, Porter PL, Tang MT, Malone KE (2009) Relationship between potentially modifiable lifestyle factors and risk of second primary contralateral breast cancer among women diagnosed with estrogen receptor-positive invasive breast cancer. J Clin Oncol 27:5312–5318
Lomen N, Johannsson O, Bendahl PO et al (1998) Steroid receptors in hereditary breast carcinomas associated with BRCA1 or BRCA2 mutations or unknown susceptibility genes. Cancer 83:310–319
Lorizio W, Rugo H, Beattie MS et al (2011) Pharmacogenetic testing affects choice of therapy among women considering tamoxifen treatment. Genome Med 3:64–75
Mann A, Miksys SL, Gaedigk A, et al (2011) The neuroprotective enzyme CYP2D6 increases in the brain with age and is lower in Parkinson’s disease patients. Neurobiol Aging Sep 27. [Epub ahead of print]
Mellick GD (2006) CYP450, genetics and Parkinson’s disease: gene x environment interactions hold the key. J Neural Transm Suppl 70:159–165
Nelson HD, Vesco KK, Haney E et al (2006) Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 295:2057–2071
Newman WG, Hadfield KD, Latif A, et al (2008) Impaired tamoxifen metabolism reduces survival in familial breast cancer patients. Clin Cancer Res 14:5913–5918
Preskorn SH, Greenblatt DJ, Flockhart D et al (2007) Comparison of duloxetine, escitalopram, and sertraline effects on cytochrome P450 2D6 function in healthy volunteers. J Clin Psychopharmacol 27:28–34
Punglia RS, Burstein HJ, Winer EP, Weeks JC (2008) Pharmacogenomic variation of CYP2D6 and the choice of optimal adjuvant endocrine therapy for postmenopausal breast cancer: a modeling analysis. J Natl Cancer Inst 100:642–648
Rae JM, Drury S, Hayes DF et al (2012) CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst 104:452–460
Reeves MD, Yawitch TM, Van der Merwe NC et al (2004) BRCA1 mutations in South African breast and/or ovarian cancer families: evidence of a novel founder mutation in Afrikaner families. Int J Cancer 110:677–682
Regan MM, Leyland-Jones B, Bouzyk M et al (2012) CYP2D6 genotype and Tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1–98 trial. J Natl Cancer Inst 104:441–451
Rosato V, Bosetti C, Talamini R et al (2011) Metabolic syndrome and the risk of breast cancer in postmenopausal women. Ann Oncol 22:2687–2692
Schroth W, Goetz MP, Hamann U et al (2009) Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA 302:1429–1436
Sluiter MD, van Rensburg EJ (2011) Large genomic rearrangements of the BRCA1 and BRCA2 genes: review of the literature and report of a novel BRCA1 mutation. Breast Cancer Res Treat 125:325–349
Van der Merwe NC, van Rensburg EJ (2009) Hereditary breast/ovarian cancer and BRCA mutations: a South African perspective. Curr Oncol 16:347
Van der Merwe NC, Hamel N, Schneider S-R et al (2012) A founder BRCA2 mutation in non-Afrikaner breast cancer patients of the Western Cape of South Africa. Clin Genet 81:179–184
Vorobiof DA, Sitas F, Vorobiof G (2001) Breast cancer incidence in South Africa. J Clin Oncol 19:125S–127S
Wright GE, Niehaus DJ, Drögemöller BI et al (2010) Elucidation of CYP2D6 genetic diversity in a unique African population: implications for the future application of pharmacogenetics in the Xhosa population. Ann Hum Genet 74:340–350
Acknowledgments
The authors gratefully acknowledge the financial support provided by the Cancer Association of South Africa (CANSA), the Harry Crossley Foundation and the National Research Foundation (NRF disclaimer: Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regards thereto). Nicole van der Merwe received a study bursary from the Technology for Innovation Agency (TIA). LR Fisher is thanked for research assistance. Dr Helen Muir is acknowledged for patient referral during the implementation phase of this translational research study, following attendance of the 6th Applied Genetics Workshop in October 2011 where the initial results obtained in the validation data set were presented. Dr Frans Cronje is acknowledged for assisting with the clinical integration of the data.
Conflict of interest
Prof MJ Kotze is a director and shareholder of Gknowmix (Pty) Ltd. that has developed a database tool for research translation under the auspices of the Innovation Centre of the South African Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
van der Merwe, N., Bouwens, C.S.H., Pienaar, R. et al. CYP2D6 genotyping and use of antidepressants in breast cancer patients: test development for clinical application. Metab Brain Dis 27, 319–326 (2012). https://doi.org/10.1007/s11011-012-9312-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-012-9312-z