Abstract
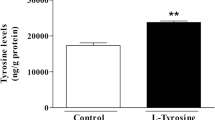
Tyrosine levels are abnormally elevated in tissues and physiological fluids of patients with inborn errors of tyrosine catabolism especially in tyrosinemia type II which is caused by deficiency of tyrosine aminotransferase (TAT) and provokes eyes, skin and central nervous system disturbances. We have recently reported that tyrosine promoted oxidative stress in vitro but the exact mechanisms of brain damage in these disorder are poorly known. In the present study, we investigated the in vivo effect of L-tyrosine (500 mg/Kg) on oxidative stress indices in cerebral cortex homogenates of 14-day-old Wistar rats. A single injection of L-tyrosine decreased glutathione (GSH) and thiol-disulfide redox state (SH/SS ratio) while thiobarbituric acid-reactive substances, protein carbonyl content and glucose-6-phosphate dehydrogenase activity were enhanced. In contrast, the treatment did not affect ascorbic acid content, and the activities of superoxide dismutase, catalase and glutathione peroxidase. These results indicate that acute administration of L-tyrosine may impair antioxidant defenses and stimulate oxidative damage to lipids and proteins in cerebral cortex of young rats in vivo. This suggests that oxidative stress may represent a pathophysiological mechanism in hypetyrosinemic patients.

Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Anderson ME (1998) Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 111–112:1–14
Bongiovanni R, Yamamoto BK, Simpson C, Jaskiw GE (2003) Pharmacokinetics of systemically administered tyrosine: a comparison of serum, brain tissue and in vivo microdialysate levels in the rat. J Neurochem 87:310–317
Browne RW, Armstrong D (1998) Reduced glutathione and glutathione disulfide. Methods Mol Biol 108:347–352
Ellaway CJ, Holme E, Standing S (2001) Outcome of tyrosinemia type III. J Inherit Metab Dis 24:824–832
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421
Goldsmith LA, Kang E, Bienfang DC, Jimbow K, Gerald P, Baden HP (1973) Tyrosinemia with plantar and palmar keratosis and keratitis. J Pediatr 83:798–805
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18:685–716
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J. Neurochem 97:1634–1658
Halliwell B, Gutteridge JMC (1985) Oxygen radicals and the nervous system. Trends Neurosci 8:22–26
Held PK (2006) Disorders of tyrosine catabolism. Mol Genet Metab 88:103–106
Kletzien RF, Harris PK, Foellmi LA (1994) Glucose-6-phosphate dehydrogenase: a “housekeeping” enzyme subject to tissue-specific regulation by hormones, nutrients, and oxidant stress. FASEB J 8:174–181
Lemonnier F, Charpentier C, Odievre M, Larregue M, Lemonnier A (1979) Tyrosine aminotransferase isoenzyme deficiency. J Pediatr 94:931–932
Leong SF, Clark JB (1984) Regional enzyme development in rat brain. Enzymes associated with glucose utilization. Biochem J. 218:131–138
Light IJ, Sutherland JM, Berry HK (1973) Clinical significance of tyrosinemia of prematurity. Am J Dis Child 125:243–247
Llesuy SF, Milei J, Molina H, Boveris A, Milei S (1985) Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4′-epiadriamycin in mice. Tumori 71:241–249
Lock EA, Gaskin P, Ellis MK, Provan WM, Robinson M, Smith LL, Prisbylla MP, Mutter LC (1996) Tissue distribution of 2-(2-nitro-4-trifluoromethylbenzoyl)cyclohexane-1-3-dione (NTBC): effect on enzymes involved in tyrosine catabolism and relevance to ocular toxicity in the rat. Toxicol Appl Pharmacol 141:439–447
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Macsai MS, Schwartz TL, Hinkle D, Hummel MB, Mulhern MG, Rootman D (2001) Tyrosinemia type II: nine cases of ocular signs and symptoms. Am J Ophthalmol 132:522–527
Mamunes P, Prince PE, Thornton NH, Hunt PA, Hitchcock ES (1976) Intellectual deficits after transient tyrosinemia in the term neonate. Pediatrics 57:675–680
Marklund SL (1985) Pyrogallol autoxidation. In: Greenwald RA (ed) Handbook of methods for oxygen radical research. CRC Press, Boca Raton, pp 243–247
Masurel-Paulet A, Poggi-Bach J, Rolland MO, Bernard O, Guffon N, Dobbelaere D, Sarles J, de Baulny HO, Touati G (2008) NTBC treatment in tyrosinaemia type I: long-term outcome in French patients. J Inherit Metab Dis 31:81–87
Mitchell GA, Grompe M, Lambert M, Tanguay RM (2001) Hypertyrosinemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. McGraw-Hill, New York, pp 1977–1982
Moriarty-Craige SE, Jones DP (2004) Extracellular thiols and thiol/disulfide redox in metabolism. Annu Rev Nutr 24:481–509
Morre MC, Hefti F, Wurtman RJ (1980) Regional tyrosine levels in rat brain after tyrosine administration. J Neural Transm 49:45–50
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Omaye ST, Turnbull JD, Sauberlich HE (1979) Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol 62:3–11
Rabinowitz LG, Williams LR, Anderson CE, Mazur A, Kaplan P (1995) Painful keratoderma and photophobia: hallmarks of tyrosinemia type II. J Pediatr 126:266–269
Reznick AZ, Parker L (1993) Free radicals and antioxidants in muscular neurological diseases and disorders. In: Poli G, Albano E, Dianzani MU (eds) Free radicals: from basic science to medicine. Birkäuser Verlag, Basel, pp 425–437
Reznick AZ, Packer L (1994) Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 233:357–363
Rice ME, Russo-Menna I (1998) Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience 82:1213–1223
Rice DN, Houston IB, Lyon IC, Macarthur BA, Mullins PR, Veale AM, Guthrie R (1989) Transient neonatal tyrosinaemia. J Inherit Metab Dis 12:13–22
Russo PA, Mitchell GA, Tanguay RM (2001) Tyrosinemia: a review. Pediatr Dev Pathol 4:212–221
Sgaravatti AM, Vargas BA, Zandoná BR, Deckmann KB, Rockenbach FJ, Moraes TB, Monserrat JM, Sgarbi MB, Pederzolli CD, Wyse ATS, Wannmacher CMD, Wajner M, Dutra-Filho CS (2008) Tyrosine promotes oxidative stress in cerebral cortex of young rats. Int J Dev Neurosci 26:551–559
Shasi Vardhan K, Pratap Rudra MP, Rao SL (1997) Inhibition of tyrosine aminotransferase by beta-N-oxalyl-L-alpha, beta-diaminopropionic acid, the Lathyrus sativus neurotoxin. J Neurochem 68:2477–2484
Stadtman ER, Levine RL (2003) Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25:207–218
Valikhani M, Akhyani M, Jafari AK, Barzegari M, Toosi S (2005) Oculocutaneous tyrosinaemia or tyrosinaemia type 2: a case report. J Eur Acad Dermatol Venereol 20:591–594
Wajner M, Latini A, Wyse AT, Dutra-Filho CS (2004) The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J Inherit Metab Dis 27:427–448
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333
Zahler WL, Cleland WW (1968) A specific and sensitive assay for disulfides. J Biol Chem 243:716–719
Acknowledgements
This work was supported by the research grants from Programa de Núcleos de Excelência (PRONEX), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and FINEP Rede Instituto Brasileiro de Neurociência (IBN-Net #01.06.0842-00).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sgaravatti, Â.M., Magnusson, A.S., de Oliveira, A.S. et al. Tyrosine administration decreases glutathione and stimulates lipid and protein oxidation in rat cerebral cortex. Metab Brain Dis 24, 415–425 (2009). https://doi.org/10.1007/s11011-009-9153-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-009-9153-6