Abstract
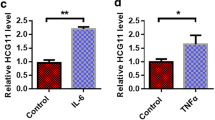
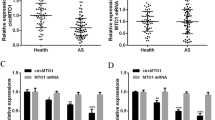
This study aimed to decipher the mechanism of circular ribonucleic acids (circRNAs) in lower extremity arteriosclerosis obliterans (LEASO). First, bioinformatics analysis was performed for screening significantly down-regulated cardiac specific circRNA—circHAT1 in LEASO. The expression of circHAT1 in LEASO clinical samples was detected by quantitative real-time polymerase chain reaction (qRT-PCR). The protein expression of splicing factor arginine/serine-rich 1 (SFRS1), α-smooth muscle actin (α-SMA), Calponin (CNN1), cyclin D1 (CNND1) and smooth muscle myosin heavy chain 11 (SMHC) in vascular smooth muscle cells (VSMCs) was detected by Western blotting. Cell Counting Kit-8 (CCK-8), 5-ethynyl-2ʹ-deoxyuridine (EdU) and Transwell assays were used to evaluate cell proliferation and migration, respectively. RNA immunoprecipitation (RNA-IP) and RNA pulldown verified the interaction between SFRS1 and circHAT1. By reanalyzing the dataset GSE77278, circHAT1 related to VSMC phenotype conversion was screened, and circHAT1 was found to be significantly reduced in peripheral blood mononuclear cells (PBMCs) of LEASO patients compared with healthy controls. Knockdown of circHAT1 significantly promoted the proliferation and migration of VSMC cells and decreased the expression levels of contractile markers. However, overexpression of circHAT1 induced the opposite cell phenotype and promoted the transformation of VSMCs from synthetic to contractile. Besides, overexpression of circHAT1 inhibited platelet-derived growth factor-BB (PDGF-BB)-induced phenotype switch of VSMC cells. Mechanistically, SFRS1 is a direct target of circHAT1 to mediate phenotype switch, proliferation and migration of VSMCs. Overall, circHAT1 regulates SFRS1 to inhibit the cell proliferation, migration and phenotype switch of VSMCs, suggesting that it may be a potential therapeutic target for LEASO.





Similar content being viewed by others
Data availability
The data that support the findings of this study are openly available in GEO database at https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE77280 and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL19978.
References
He Y, Xie C, Xia Y, Rang J, Peng L (2021) Humanistic care interventions in patients with lower extremity arteriosclerosis obliterans. Am J Transl Res 13:10527–10535
Chen Z, Wang M, Huang K, He Q, Li H, Chang G (2018) MicroRNA-125b affects vascular smooth muscle cell function by targeting serum response factor. Cell Physiol Biochem 46:1566–1580. https://doi.org/10.1159/000489203
Libby P, Bornfeldt KE, Tall AR (2016) Atherosclerosis: successes, surprises, and future challenges. Circ Res 118:531–534. https://doi.org/10.1161/CIRCRESAHA.116.308334
Wang HY, Liu T, Chen Y (2017) Atherosclerosis: current understanding and future challenges. Med J West Chin 29:11–13
Koshikawa M, Ikeda U (2010) Arteriosclerosis obliterans (ASO). Nihon Rinsho 68:926–929
Chistiakov DA, Orekhov AN, Bobryshev YV (2015) Vascular smooth muscle cell in atherosclerosis. Acta Physiol (Oxf) 214:33–50. https://doi.org/10.1111/apha.12466
Owens GK, Kumar MS, Wamhoff BR (2004) Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84:767–801. https://doi.org/10.1152/physrev.00041.2003
Bennett MR, Sinha S, Owens GK (2016) Vascular smooth muscle cells in atherosclerosis. Circ Res 118:692–702. https://doi.org/10.1161/CIRCRESAHA.115.306361
Hu DJ, Li ZY, Zhu YT, Li CC (2020) Overexpression of long noncoding RNA ANRIL inhibits phenotypic switching of vascular smooth muscle cells to prevent atherosclerotic plaque development in vivo. Aging (Albany NY) 13:4299–4316. https://doi.org/10.18632/aging.202392
Chen LL, Yang L (2015) Regulation of circRNA biogenesis. RNA Biol 12:381–388. https://doi.org/10.1080/15476286.2015.1020271
Gomes CPC, Salgado-Somoza A, Creemers EE, Dieterich C, Lustrek M, Devaux Y et al (2018) Circular RNAs in the cardiovascular system. Noncoding RNA Res 3:1–11. https://doi.org/10.1016/j.ncrna.2018.02.002
Fu LY, Hu YR, Guo JM (2015) CircRNAs and human diseases. Chin J Biochem Mol Biol 31:771–778. https://doi.org/10.13865/j.cnki.cjbmb.2015.08.01
Shao Y, Chen Y (2016) Roles of circular RNAs in neurologic disease. Front Mol Neurosci 9:25. https://doi.org/10.3389/fnmol.2016.00025
Zhou R, Wu Y, Wang W, Su W, Liu Y, Wang Y et al (2018) Circular RNAs (circRNAs) in cancer. Cancer Lett 425:134–142. https://doi.org/10.1016/j.canlet.2018.03.035
Zhou Z, Sun B, Huang S, Zhao L (2019) Roles of circular RNAs in immune regulation and autoimmune diseases. Cell Death Dis 10:503. https://doi.org/10.1038/s41419-019-1744-5
Ma N, Zhang W, Wan J (2020) Research progress on circRNA in nervous system diseases. Curr Alzheimer Res 17:687–697. https://doi.org/10.2174/1567205017666201111114928
Wang K, Tan G, Tian R, Zhou H, Xiang C, Pan K (2022) Circular RNA circ_0021001 regulates miR-148b-3p/GREM1 axis to modulate proliferation and apoptosis of vascular smooth muscle cells. Metab Brain Dis 37:2027–2038. https://doi.org/10.1007/s11011-022-01014-4
Ye M, Ni Q, Wang H, Wang Y, Yao Y, Li Y et al (2023) CircRNA circCOL1A1 acts as a sponge of miR-30a-5p to promote vascular smooth cell phenotype switch through regulation of Smad1 expression. Thromb Haemost 123:97–107. https://doi.org/10.1055/s-0042-1757875
Liu C, Li N, Li F, Deng W, Dai G, Tang Y et al (2023) CircHIPK2 facilitates phenotypic switching of vascular smooth muscle cells in hypertension. J Hum Hypertens 37:1021–1027. https://doi.org/10.1038/s41371-023-00834-w
Tian J, Fu Y, Li Q, Xu Y, Xi X, Zheng Y et al (2020) Differential expression and bioinformatics analysis of CircRNA in PDGF-BB-induced vascular smooth muscle cells. Front Genet 11:530. https://doi.org/10.3389/fgene.2020.00530
Chen W, Lin J, Li B, Cao S, Li H, Zhao J et al (2020) Screening and functional prediction of differentially expressed circRNAs in proliferative human aortic smooth muscle cells. J Cell Mol Med 24:4762–4772. https://doi.org/10.1111/jcmm.15150
Zhang X, Wang P, Yuan K, Li M, Shen Y, Que H et al (2021) Hsa_circ_0024093 accelerates VSMC proliferation via miR-4677-3p/miR-889-3p/USP9X/YAP1 axis in in vitro model of lower extremity ASO. Mol Ther Nucleic Acids 26:511–522. https://doi.org/10.1016/j.omtn.2021.07.026
Paz S, Ritchie A, Mauer C, Caputi M (2021) The RNA binding protein SRSF1 is a master switch of gene expression and regulation in the immune system. Cytokine Growth Factor Rev 57:19–26. https://doi.org/10.1016/j.cytogfr.2020.10.008
Das S, Krainer AR (2014) Emerging functions of SRSF1, splicing factor and oncoprotein, in RNA metabolism and cancer. Mol Cancer Res 12:1195–1204. https://doi.org/10.1158/1541-7786.MCR-14-0131
Sandoval-Castellanos AM, Bhargava A, Zhao M, Xu J, Ning K (2023) Serine and arginine rich splicing factor 1: a potential target for neuroprotection and other diseases. Neural Regen Res 18:1411–1416. https://doi.org/10.4103/1673-5374.360243
Xie N, Chen M, Dai R, Zhang Y, Zhao H, Song Z et al (2017) SRSF1 promotes vascular smooth muscle cell proliferation through a Delta133p53/EGR1/KLF5 pathway. Nat Commun 8:16016. https://doi.org/10.1038/ncomms16016
He X, Lian Z, Yang Y, Wang Z, Fu X, Liu Y et al (2020) Long non-coding RNA PEBP1P2 suppresses proliferative VSMCs phenotypic switching and proliferation in atherosclerosis. Mol Ther Nucleic Acids 22:84–98. https://doi.org/10.1016/j.omtn.2020.08.013
Patel P, Ivanov A, Ramasubbu K (2016) Myocardial viability and revascularization: current understanding and future directions. Curr Atheroscler Rep 18:32. https://doi.org/10.1007/s11883-016-0582-5
Bartoschek M, Pietras K (2018) PDGF family function and prognostic value in tumor biology. Biochem Biophys Res Commun 503:984–990. https://doi.org/10.1016/j.bbrc.2018.06.106
Huang C, Huang W, Wang R, He Y (2020) Ulinastatin inhibits the proliferation, invasion and phenotypic switching of PDGF-BB-induced VSMCs via Akt/eNOS/NO/cGMP signaling pathway. Drug Des Dev Ther 14:5505–5514. https://doi.org/10.2147/DDDT.S275488
Lu QB, Wan MY, Wang PY, Zhang CX, Xu DY, Liao X et al (2018) Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFkappaB/mTOR/P70S6K signaling cascade. Redox Biol 14:656–668. https://doi.org/10.1016/j.redox.2017.11.012
Wang C, Liu Y, He D (2019) Diverse effects of platelet-derived growth factor-BB on cell signaling pathways. Cytokine 113:13–20. https://doi.org/10.1016/j.cyto.2018.10.019
Altesha MA, Ni T, Khan A, Liu K, Zheng X (2019) Circular RNA in cardiovascular disease. J Cell Physiol 234:5588–5600. https://doi.org/10.1002/jcp.27384
Anczukow O, Rosenberg AZ, Akerman M, Das S, Zhan L, Karni R et al (2012) The splicing factor SRSF1 regulates apoptosis and proliferation to promote mammary epithelial cell transformation. Nat Struct Mol Biol 19:220–228. https://doi.org/10.1038/nsmb.2207
Sanford JR, Wang X, Mort M, Vanduyn N, Cooper DN, Mooney SD et al (2009) Splicing factor SFRS1 recognizes a functionally diverse landscape of RNA transcripts. Genome Res 19:381–394. https://doi.org/10.1101/gr.082503.108
Tripathi V, Song DY, Zong X, Shevtsov SP, Hearn S, Fu XD et al (2012) SRSF1 regulates the assembly of pre-mRNA processing factors in nuclear speckles. Mol Biol Cell 23:3694–3706. https://doi.org/10.1091/mbc.E12-03-0206
Funding
This study has been supported by Guangdong Natural Science Foundation (2021A1515011173); Guangdong Province Medical Science and Technology Research Project (A2020451).
Author information
Authors and Affiliations
Contributions
XH and FF conceptualized and designed the study, drafted the initial manuscript. KQ, KQ, SC, WW, YL and WC collected the data and carried out the initial analyses. ZZ and SH critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethical approval
The study was made based on the Declaration of Helsinki with the approval by the Research Ethics Committee of Southern Hospital of Southern Medical University.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, Xy., Fu, Fy., Qian, K. et al. CircHAT1 regulates the proliferation and phenotype switch of vascular smooth muscle cells in lower extremity arteriosclerosis obliterans through targeting SFRS1. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04932-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04932-2