Abstract
The human immune system responds to harmful foreign invaders frequently encountered by the body and employs defense mechanisms to counteract such assaults. Various exogenous and endogenous factors play a prominent role in maintaining the balanced functioning of the immune system, which can result in immune suppression or immune stimulation. With the advent of different immune-modulatory agents, immune responses can be modulated or regulated to control infections and other health effects. Literature provides evidence on various immunomodulators from different sources and their role in modulating immune responses. Due to the limited efficacy of current drugs and the rise in drug resistance, there is a growing need for new therapies for infectious diseases. In this review, we aim to provide a comprehensive overview of different immune-modulating agents and immune therapies specifically focused on viral infectious diseases.
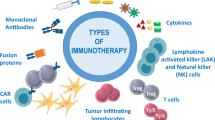
Graphical Abstract








Similar content being viewed by others
Data availability
The authors declared that the research data referred to correctly cited in the manuscript's reference section.
References
Baxter D (2007) Active and passive immunity, vaccine types, excipients and licensing. Occup Med 57(8):552–556
Vesely MD, Kershaw MH, Schreiber RD et al (2011) Natural innate and adaptive immunity to cancer. Annu Rev Immunol 29:235–271
Florez-Sampedro L, Song S, Melgert BN (2018) The diversity of myeloid immune cells shaping wound repair and fibrosis in the lung. Regeneration 5(1):3–25
Puri A, Saxena R, Saxena RP et al (1994) Immunostimulant activity of Nyctanthes arbor-tristis L. J Ethnopharmacol 42(1):31–37
Jantan I, Ahmad W, Bukhari SN (2015) Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front Plant Sci 25(6):655
Wen CC, Chen HM, Yang NS (2012) Developing phytocompounds from medicinal plants as immunomodulators. Adv Bot Res 62:197–272. https://doi.org/10.1016/B978-0-12-394591-4.00004-0
Shashank K, Abhay KP (2013) Chemistry and biological activities of flavonoids: an overview. Sci World J 2013:1–6
Kumar S, Gupta P, Sharma S et al (2011) A review on immunostimulatory plants. Chin J Integr Med 9(2):117–128
Germain RN (2010) Vaccines and the future of human immunology. Immunity 33(4):441–450
Brotherton JM (2015) HPV prophylactic vaccines: lessons learned from 10 years experience. Future Virol 10(8):999–1009
Frazer IH (2014) Development and implementation of papillomavirus prophylactic vaccines. J Immunol Res 192(9):4007–4011
Plotkin SA (2003) Vaccines, vaccination, and vaccinology. JInfect Dis 187(9):1349–1359
James E. CroweJr. Prevention of fetal and early life infections through maternal–neonatal immunization. In: Remington, Klien, Wilson, Nizet, Maldonado Eds. 7th ed. Philadelphia, PA: Saunders/Elsevier; 2011.pp.1212–1230
Coffman RL, Sher A, Seder RA (2010) Vaccine adjuvants: putting innate immunity to work. Immunity 33(4):492–503
Pasquale AD, Preiss S, Silva FT et al (2015) Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines 3(2):320–343
Nicholls EF, Madera L, Hancock RE (2010) Immunomodulators as adjuvants for vaccines and antimicrobial therapy. Ann N Y Acad Sci 1213(1):46–61
Ghimire TR, Benson RA, Garside P et al (2012) Alum increases antigen uptake, reduces antigen degradation and sustains antigen presentation by DCs in vitro. Immunol Lett 147(1–2):55–62
Vogel F. Immunologic adiuvants. Vaccines: Expert Consult. 2008:59–71.
Marrack P, McKee AS, Munks MW (2009) Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol 9(4):287–293
Grun JL, Maurer PH (1989) Different T helper cell subsets elicited in mice utilizing two different adjuvant vehicles: the role of endogenous interleukin 1 in proliferative responses. Cell Immunol 121(1):134–145
Metcalf D (2013) The colony-stimulating factors and cancer. Cancer Immunol Res 1(6):351–356
Metcalf D, Nicola NA (1995) The hematopoietic colony-stimulating factors: From Biology to Clinical Applications. Cambridge University Press, Cambridge, UK, pp 1–327
Chuang YM, He L, Pinn ML et al (2021) Albumin fusion with granulocyte-macrophage colony-stimulating factor acts as an immunotherapy against chronic tuberculosis. Cell Mol Immunol 18(10):2393–2401
O’Hagan DT, Ott GS, De Gregorio E, Seubert A (2012) The mechanism of action of MF59—an innately attractive adjuvant formulation. Vaccine 30:4341–4348. https://doi.org/10.1016/j.vaccine.2011.09.061
O’Hagan DT, Friedland LR, Hanon E, Didierlaurent AM (2017) Towards an evidence based approach for the development of adjuvanted vaccines. Curr Opin Immunol 47:93–102. https://doi.org/10.1016/j.coi.2017.07.010
Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T et al (2009) Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A 106:7962–7967. https://doi.org/10.1073/pnas.0903181106
O’Hagan DT, Rappuoli R, De Gregorio E, Tsai T, Del Giudice G (2011) MF59 adjuvant: the best insurance against influenza strain diversity. Expert Rev Vaccines 10:447–462. https://doi.org/10.1586/erv.11.23
Ko EJ, Kang SM (2018) Immunology and efficacy of MF59-adjuvanted vaccines. Hum Vaccin Immunother 14(12):3041–3045
Kayraklioglu N, Horuluoglu B, Klinman DM (2021) CpG oligonucleotides as vaccine adjuvants. Methods Mol Biol 2197:51–85
Shirota H, Klinman DM (2014) Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev Vaccines 13:299–312. https://doi.org/10.1586/14760584.2014.863715
Wang S, Han Q, Zhang G et al (2011) CpG oligodeoxynucleotide-adjuvanted fusion peptide derived from HBcAg epitope and HIV-Tat may elicit favorable immune response in PBMCs from patients with chronic HBV infection in the immunotolerant phase. Int Immunopharmacol 11:406–411
Duggal S, Chugh TD, Duggal AK (2012) HIV and malnutrition: effects on immune system. ClinDev Immunol. https://doi.org/10.1155/2012/784740
Sachan S, Dhama K, Latheef SK, et al. Immunomodulatory Potential of Tinospora cordifolia and CpG ODN [TLR21 Agonist] against the very virulent, infectious Bursal disease virus in SPF Chicks. Vaccines. 2019;7[3]:106.
Seirafianpour F, Mozafarpoor S, Fattahi N et al (2020) Treatment of COVID-19 with pentoxifylline: Could it be a potential adjuvant therapy? Dermatol Ther. https://doi.org/10.1111/dth.13733
Gensler LS (2013) Glucocorticoids: complications to anticipate and prevent. The Neurohospitalist 3(2):92–97
Coutinho AE, Chapman KE (2011) The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335(1):2–13
Stockman LJ, Bellamy R, Garner P (2006) SARS: systematic review of treatment effects. PLoS Med 3(9):1525–1531
Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., Almotairi A., Al Khatib K., Alraddadi B., Shalhoub S., Abdulmomen A., Qushmaq I., Mady A., Solaiman O., Al-Aithan A.M., Al-Raddadi R., Ragab A., Balkhy H.H., Al Harthy A., Deeb A.M., Al Mutairi H., Al-Dawood A., Merson L., Hayden F.G., Fowler R.A., Saudi Critical Care Trial G. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am. J. Respir. Crit. Care Med. 2018;197(6):757–767.
Kudo K, Takasaki J, Manabe T, Uryu H, Yamada R, Kuroda E, Kobayashi N, Matsushita T (2012) Systemic corticosteroids and early administration of antiviral agents for pneumonia with acute wheezing due to influenza A(H1N1)pdm09 in Japan. PLoS ONE. https://doi.org/10.1371/journal.pone.0032280
Romanelli A, Mascolo S (2020) Immunosuppression drug-related and clinical manifestation of Coronavirus disease 2019: a therapeutical hypothesis. Am J Transplant 20(7):1947–1948
Alexaki VI, Henneicke H (2021) The role of glucocorticoids in the management of COVID-19. Horm Metab Res 53(1):9–15
Galon J, Franchimont D, Hiroi N et al (2002) Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J 16:61–71
Sarzani R, Spannella F, Giulietti F, Di Pentima C, Giordano P, Giacometti A (2022) Possible harm from glucocorticoid drugs misuse in the early phase of SARS-CoV-2 infection: a narrative review of the evidence. Intern Emerg Med 17(2):329–338. https://doi.org/10.1007/s11739-021-02860-3
Strehl C, Ehlers L, Gaber T et al (2019) Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol 10:1744
Cain DW, Cidlowski JA (2017) Immune regulation by glucocorticoids. Nat Rev Immunol 17(4):233–247
Kountouras J, Zavos C, Chatzopoulos D (2004) Immunomodulatory benefits of cyclosporine A in inflammatory bowel disease. J Cell Mol Med 8(3):317–328
Russell G, Graveley R, Seid J, et al. Mechanisms of action of cyclosporine and effects on connective tissues. InSeminars in arthritis and rheumatism 1992 Jun 1 [Vol. 21, No. 6, pp. 16–22].
Matsuda S, Koyasu S (2000) Mechanisms of action of cyclosporine. Immunopharmacology 47:119–125. https://doi.org/10.1016/s0162-3109(00)00192-
Glowacka P, Rudnicka L, Warszawik-Hendzel O, Sikora M, Goldust M, Gajda P, Stochmal A, Blicharz L, Rakowska A, Olszewska M (2020) The antiviral properties of cyclosporine. Focus on coronavirus, hepatitis C virus, influenza virus, and human immunodeficiency virus infections. Biology (Basel) 9(8):192. https://doi.org/10.3390/biology9080192
Wilde AH, Zevenhoven-Dobbe JC, van der Meer Y, Thiel V, Narayanan K, Makino S, Snijder EJ, van Hemert MJ (2011) Cyclosporin A inhibits the replication of diverse coronaviruses. J Gen Virol. https://doi.org/10.1099/vir.0.034983-0
Fenizia C, Galbiati S, Vanetti C, Vago R, Clerici M, Tacchetti C, Daniele T (2022) Cyclosporine a inhibits viral infection and release as well as cytokine production in lung cells by three SARS-CoV-2 variants. Microbiol Spectr. https://doi.org/10.1128/spectrum.01504-21
Williams JW, Xiao F, Foster PF, et al. Immunosuppressive effects of leflunomide in a cardiac allograft model. InTransplantation proceedings 1993 [Vol. 25, No. 1, pp. 745–746].
Kuchie CA, Thoenes GH, Langer KH, et al Prevention of kidney and skin graft rejection in rats by leflunomide, a new immunomodulating agent. In Transplantation proceedings 1991 [Vol. 23, No. 1, pp. 1083–1086].
Dimitrova P, Kalden JR, Schulze-Koops H (2002) Leflunomide: an immunosuppressive drug with multiple effects on T cell function. Mod Rheumatol 12(3):195–200
Van der Heijden EH, Hartgring SA et al (2019) Additive immunosuppressive effect of leflunomide and hydroxychloroquine supports rationale for combination therapy for Sjögren’s syndrome. Expert Rev Clin Immunol 15(7):801–808
Moliva JI, Turner J, Torrelles JB (2017) Immune responses to bacillus Calmette-Guérin vaccination: why do they fail to protect against Mycobacterium tuberculosis? Front Immunol 8:407
Read SW, DeGrezia M, Ciccone EJ, DerSimonian R, Higgins J, Adelsberger JW, Starling JM, Rehm C, Sereti I (2010) The effect of leflunomide on cycling and activation of T-cells in HIV-1-infected participants. PLoS ONE. https://doi.org/10.1371/journal.pone.0011937
Alamri RD, Elmeligy MA, Albalawi GA, Alquayr SM, Alsubhi SS, El-Ghaiesh SH (2021) Leflunomide an immunomodulator with antineoplastic and antiviral potentials but drug-induced liver injury: A comprehensive review. Int Immunopharmacol. https://doi.org/10.1016/j.intimp.2021.107398
M. Millis, L. Perea, Leflunomide in Mild COVID-19 Patients. In.: https://ClinicalTrials.gov/show/NCT04361214, 2020
Mattar T, Kochhar K, Bartlett R, Bremer EG, Finnegan A (1993) Inhibition of the epidermal growth factor receptor tyrosine kinase activity by leflunomide. FEBS Lett 334(2):161–164
Chacko B, John GT (2012) Leflunomide for cytomegalovirus: bench to bedside. Transplant Infect Dis. 14(2):111–120
Pandey S, Cabot PJ, Shaw PN, Hewavitharana AK. Anti-inflammatory and immunomodulatory properties of Carica papaya. J Immunotoxicol. 2016 ;13(4):590–602. doi: https://doi.org/10.3109/1547691X.2016.1149528. Epub 2016 Jul 14. PMID: 27416522 and documented to be effective against dengue virus
Ahmad N, Fazal H, Ayaz M, Abbasi BH, Mohammad I, Fazal L (2011) Dengue fever treatment with Carica papaya leaves extracts. Asian Pac J Trop Biomed 1(4):330–333. https://doi.org/10.1016/S2221-1691(11)60055-5
Lalani S, Poh CL. Flavonoids as antiviral agents for Enterovirus A71 [EV-A71]. Viruses. 2020; 12[2]:184.
Mendes LF, Gaspar VM, Conde TA, et al. Flavonoid-mediated immunomodulation of human macrophages involves key metabolites and metabolic pathways. Sci Rep. 2019; 9[1]:1.
Middleton E, Kandaswami C, Theoharides TC (2000) The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev 52(4):673–751
Sun X, Yamasaki M, Katsube T et al (2015) Effects of quercetin derivatives from mulberry leaves: Improved gene expression related hepatic lipid and glucose metabolism in short-term high-fat fed mice. Nutr Res Pract 9(2):137–143
Middleton E Jr (1998) Effect of plant flavonoids on immune and inflammatory cell function. Adv Exp Med Biol 439:175–182. https://doi.org/10.1007/978-1-4615-5335-9_13
Hosseinzade A, Sadeghi O, Naghdipour Biregani A et al (2019) Immunomodulatory effects of flavonoids: possible induction of T CD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front Immunol 10:51
Schwartz A, Sutton SL, Middleton E Jr (1982) Quercetin inhibition of the induction and function of cytotoxic T lymphocytes. Immunopharmacol 4(2):125–138
Rogerio AP, Dora CL, Andrade EL, Chaves JS, Silva LF, Lemos-Senna E, Calixto JB (2010) Anti-inflammatory effect of quercetin-loaded microemulsion in the airways allergic inflammatory model in mice. Pharmacol Res 61(4):288–297
Chirumbolo S (2010) The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets 9(4):263–285
Lee KM, Hwang MK, Lee DE, Lee KW, Lee HJ (2010) Protective effect of quercetin against arsenite-induced COX-2 expression by targeting PI3K in rat liver epithelial cells. J Agric Food Chem 58:5815–5820
Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, Liu H, Yin Y (2016) Quercetin, Inflammation and Immunity. Nutrients 8(3):167
Doishorobi FM, Nisa FY, Saha S, Chowdhury MAH, Srisuphanunt M, Hossain KH, Rahman MA (2023) Quercetin: a functional food-flavonoid incredibly attenuates emerging and re-emerging viral infections through immunomodulatory actions. Molecules 28(3):938. https://doi.org/10.3390/molecules28030938
DiNicolantonio JJ, McCarty MF (2020) Targeting Casein kinase 2 with quercetin or enzymatically modified isoquercitrin as a strategy for boosting the type 1 interferon response to viruses and promoting cardiovascular health. Med Hypotheses 142:109800
Komaravelli N, Kelley JP, Garofalo MP, Wu H, Casola A, Kolli D (2015) Role of dietary antioxidants in human metapneumovirus infection. Virus Res 200:19–23
Di Pierro F, Derosa G, Maffioli P, Bertuccioli A, Togni S, Riva A, Allegrini P, Khan A, Khan S, Khan BA et al (2021) Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int J Gen Med 14:2359–2366
Te Velthuis, A.J.; Fodor, E. Influenza virus RNA polymerase: Insights into the mechanisms of viral RNA synthesis. Nat. Rev Microbiol. 2016, 14, 479–493. [CrossRef] [PubMed]
Parvez, M.K.; Rehman, T.; Alam, P.; Al-Dosari, M.S.; Alqasoumi, S.I.; Alajmi, M.F. Plant-derived antiviral drugs as novel hepatitisB virus inhibitors: Cell culture and molecular docking study. Saudi Pharm. J. 2018, 27, 389–400. [CrossRef] [PubMed]
Yang, X.; Zhu, X.; Ji, H.; Deng, J.; Lu, P.; Jiang, Z.; Li, X.;Wang, Y.;Wang, C.; Zhao, J.; et al. Quercetin synergistically reactivateshuman immunodeficiency virus type 1 latency by activating nuclear factor-_B. Mol. Med. Rep. 2017, 17, 2501–2508. [CrossRef]
Fatima K, Mathew S, Suhail M, Ali A, Damanhouri G, Azhar E, Qadri I (2014) Docking studies of Pakistani HCV NS3 helicase: Apossible antiviral drug target. PLoS ONE 9:e106339
Hung, P.-Y.; Ho, B.-C.; Lee, S.-Y.; Chang, S.-Y.; Kao, C.-L.; Lee, S.-S.; Lee, C.-N. Houttuynia cordata Targets the Beginning Stage ofHerpes Simplex Virus Infection. PLoS ONE 2015, 10, e0115475. [CrossRef] [PubMed].
Liu HJ, Fan YL, Liao HH et al (2017) Apigenin alleviates STZ-induced diabetic cardiomyopathy. Mol Cell Bio chem 428(1–2):9–21
Critchfield JW, Butera ST, Folks TM (1996) Inhibition of HIV activation in latently infected cells by flavonoid compounds. AIDS Res Hum Retroviruses 12(1):39–46
Shibata C, Ohno M, Otsuka M, Kishikawa T, Goto K, Muroyama R, Kato N, Yoshikawa T, Takata A, Koike K (2014) The flavonoid apigenin inhibits hepatitis C virus replication by decreasing mature microRNA122 levels. Virology 462–463:42–48. https://doi.org/10.1016/j.virol.2014.05.024. (Epub 2014 Jun 14 PMID: 25092460)
Lv X, Qiu M, Chen D, Zheng N, Jin Y, Wu Z (2014) Apigenin inhibits enterovirus 71 replication through suppressing viral IRES activity and modulating cellular JNK pathway. Antiviral Res 109:30–41. https://doi.org/10.1016/j.antiviral.2014.06.004. (Epub 2014 Jun 24 PMID: 24971492)
Zhang W, Qiao H, Lv Y, Wang J, Chen X, Hou Y, Tan R, Li E. Apigenin inhibits enterovirus-71 infection by disrupting viral RNA association with trans-acting factors. PLoS One. 2014 Oct 16;9(10):e110429. doi: https://doi.org/10.1371/journal.pone.0110429. PMID: 25330384; PMCID: PMC4199717.-
.Xu X, Miao J, Shao Q, Gao Y, Hong L. (2020) Apigenin suppresses influenza A virus-induced RIG-I activation and viral replication. J Med Virol 92(12):3057–3066
Acchioni C, Acchioni M, Mancini F, Amendola A, Marsili G, Tirelli V, Gwee CP, Chan KW, Sandini S, Bisbocci M, Mysara M, ElHefnawi M, Sanchez M, Venturi G, Barreca ML, Manfroni G, Bresciani A, Vasudevan SG, Sgarbanti M (2023) A cellular screening platform, stably expressing DENV2 NS5, defines a novel anti-DENV mechanism of action of Apigenin based on STAT2 activation. Virology 583:1–13. https://doi.org/10.1016/j.virol.2023.03.016. (Epub 2023 Apr 5 PMID: 37060797)
bdizadeh R, Hadizadeh F, Abdizadeh T. Evaluation of apigenin-based biflavonoid derivatives as potential therapeutic agents against viral protease (3CLpro) of SARS-CoV-2 via molecular docking, molecular dynamics and quantum mechanics studies. J BiomolStructDyn. 2022. doi: https://doi.org/10.1080/07391102.2022.2098821
Cardenas H, Arango D, Nicholas C, Duarte S, Nuovo GJ, He W, et al. Dietary apigenin exerts immune-regulatory activity in vivo by reducing NF-κB activity, halting leukocyte infiltration and restoring normal metabolic function. Int J Mol Sci. 2016;17[3]:323.
Wang J, Liu YT, Xiao L, Zhu L et al (2014) Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 37(6):2085–2090
Che DN, Cho BO, Shin JY, Kang HJ, Kim Shukla S, Bajpai VK, Kim M (2014) Plants as potential sources of natural immunomodulators. Rev Environ SciBiotechnol 13(1):17–33
Roszkowski W, Roszkowski K, Ko HL et al (1990) Immunomodulation by propionibacteria. Zentralblatt für Bakteriologie 274(3):289–298
Marinova S, Tchorbadjiiska L, Petrunov B et al (2000) Immunostimulating and protective effects of an oral polybacterial immunomodulator ‘Dentavax’in a rabbit experimental model. Int J Immunopharmacol 22(11):843–854
Talib WH, Saleh S. Propionibacterium acnes augments antitumor, anti-angiogenesis and immunomodulatory effects of melatonin on breast cancer implanted in mice. PloS one. 2015;10[4]:e0124384.
Frediansyah, A.; Sofyantoro, F.; Alhumaid, S.; Al Mutair, A.; Albayat, H.; Altaweil, H.I.; Al-Afghani, H.M.; AlRamadhan, A.A.; AlGhazal, M.R.; Turkistani, S.A.; Abuzaid, A.A.; Rabaan, A.A. Microbial natural products with antiviral activities, including anti-SARS-CoV-2: a review. Molecules 2022, 27, 4305. https://doi.org/10.3390/molecules27134305
Galdeano CM, Perdigon G (2004) Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J Appl Microbiol 97(4):673–681
Foligné B, Dewulf J, Breton J et al (2010) Probiotic properties of non-conventional lactic acid bacteria: immunomodulation by Oenococcus oeni. Int J Food Microbiol 140(2–3):136–145
Bodera P, Chcialowski A (2009) Immunomodulatory effect of probiotic bacteria. Recent Pat Inflamm Allergy Drug Discov 3(1):58–64
Azad M, Kalam A, Sarker M et al (2018) Probiotic species in the modulation of gut microbiota: an overview. BioMed Res Int 2018:1–8
Jounai K, Sugimura T, Ohshio K, et al. Oral administration of Lactococcus lactis subsp. lactis JCM5805 enhances lung immune response resulting in protection from murine parainfluenza virus infection. PloS one. 2015; doi: https://doi.org/10.1371/journal.pone.0119055
Wells JM, Mercenier A (2008) Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol 6(5):349–362
Rosenberg H F., B Domachowske J. Inflammatory responses to respiratory syncytial virus (RSV) infection and the development of immunomodulatory pharmacotherapeutics. Curr Med Chem. 2012;19:1424–1431.
Baud D, Agri VD, Gibson GR, Reid G, Giannoni E (2020) Using probiotics to flatten the curve of coronavirus disease COVID-2019 pandemic. Front Public Health. https://doi.org/10.3389/fpubh.2020.00186
Kobayashi N, Saito T, Uematsu T, Kishi K, Toba M, Kohda N, Suzuki T (2011) Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. Int Immunopharmacol 11:199–203
Yasui H, Kiyoshima J, Hori T, Shida K (1999) Protection against influenza virus infection of mice fed Bifidobacterium breve YIT4064. Clin Diagn Lab Immunol 6:186–192
Jiao Q, Li L, Mu Q, Zhang Q (2014) Immunomodulation of nanoparticles in nanomedicine applications. Biomed Res Int 2014:426028
De Nicola M, Gattia DM, Traversa E et al (2013) Maturation and demise of human primary monocytes by carbon nanotubes. J Nanopart Res 15(6):1–5
Hoshino A, Hanada S, Manabe N (2009) Immune response induced by fluorescent nanocrystal quantum dots in vitro and in vivo. IEEE Trans Nano Biosci 8(1):51–57
Müller K, Skepper JN, Posfai M et al (2007) Effect of ultrasmallsuperparamagnetic iron oxide nanoparticles [Ferumoxtran-10] on human monocyte-macrophages in vitro. Biomaterials 28(9):1629–1642
Shen CC, Liang HJ, Wang CC, Liao MH, Jan TR (2012) Iron oxide nanoparticles suppressed T helper 1 cell-mediated immunity in a murine model of delayed-type hypersensitivity. Int J Nanomedicine 7:2729–2737. https://doi.org/10.2147/IJN.S31054
Draz MS, Wang YJ, Chen FF, Xu Y, Shafiee H (2017) Electrically oscillating plasmonic nanoparticles for enhanced DNA vaccination against hepatitis C virus. Adv Funct Mater. https://doi.org/10.1002/adfm.201604139
Vetro M, Safari D, Fallarini S, Salsabila K, Lahmann M, Penadés S, Lay L, Marradi M, Compostella F (2017) Preparation and immunogenicity of gold glyco-nanoparticles as antipneumococcal vaccine model. Nanomedicine (Lond) 12(1):13–23. https://doi.org/10.2217/nnm-2016-0306
Liu Y, Wang H, Li D, Tian Y, Liu W, Zhang L, Zheng W, Hao Y, Liu J, Yang Z, Shao Y, Jiang X (2016) In situ formation of peptidic nanofibers can fundamentally optimize the quality of immune responses against HIV vaccine. Nanoscale Horiz 1(2):135–143. https://doi.org/10.1039/c5nh00064e
Deng L, Mohan T, Chang TZ, Gonzalez GX, Wang Y, Kwon YM, Kang SM, Compans RW, Champion JA, Wang BZ (2018) Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat Commun 9(1):359. https://doi.org/10.1038/s41467-017-02725-4
Yang J, Shim SM, Nguyen TQ, Kim EH, Kim K, Lim YT, Sung MH, Webby R, Poo H (2017) Poly-γ-glutamic acid/chitosan nanogel greatly enhances the efficacy and heterosubtypic cross-reactivity of H1N1 pandemic influenza vaccine. Sci Rep 7:44839. https://doi.org/10.1038/srep44839
Feng X, Xu W, Li Z, Song W, Ding J, Chen X (2019) Immunomodulatory Nanosystems. Adv Sci (Weinh). https://doi.org/10.1002/advs.201900101
Zhang G, Campbell GR, Zhang Q, Maule E, Hanna J, Gao W, Zhang L, Spector SA (2020) CD4+ t cell-mimicking nanoparticles broadly neutralize hiv-1 and suppress viral replication through autophagy. MBio 11:e45
Morris D, Ansar M, Speshock J, et al. Antiviral and immunomodulatory activity of silver nanoparticles in experimental RSV infection. Viruses. 2019 ;11[8]:732.
Kumar NG, Contaifer D, Madurantakam P, et al Dietary bioactive fatty acids as modulators of immune function: implications on human health. Nutrients. 2019;11:2974. doi: https://doi.org/10.3390/nu11122974
Parnham MJ, Englberger W. Lipid mediators and lymphocyte function. In: Michael A. Bray, John Morley (Eds) The Pharmacology of Lymphocytes 1988 [pp. 385–414]. Springer, Berlin Heidelberg.
Anderson M, Fritsche KL (2002) [n-3] Fatty acids and infectious disease resistance. J Nutr 132(12):3566–3576
Shapiro H, Lev S, Cohen J et al (2009) Polyphenols in the prevention and treatment of sepsis syndromes: rationale and pre-clinical evidence. Nutrition 25(10):981–997
Khan NA (2010) Polyunsaturated fatty acids in the modulation of T-cell signalling. Prostaglandins Leukot Essent Fatty Acids 82(4–6):179–187
Husson MO, Ley D, Portal C et al (2016) Modulation of host defence against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J Infect 73(6):523–535. https://doi.org/10.1016/j.jinf.2016.10.001
Hancock RE, Nijnik A, Philpott DJ (2012) Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol 10(4):243–254
Nijnik A (2013) Immunomodulatory approaches for prevention and treatment of infectious diseases. Curr Opin Microbiol 16(5):590–595
Masihi KN (2001) Fighting infection using immunomodulatory agents. Expert Opin Biol Ther 1(4):641–653
Shapiro H, Lev S, Cohen J, Singer P (2009) Polyphenols in the prevention and treatment of sepsis syndromes: rationale and pre-clinical evidence. Nutrition 25(10):981–997
Tas SW, Baeten DL (2016) Recent advances in the treatment of immune-mediated inflammatory diseases. Methods Mol Biol 1371:143–155
Bedoui Y, Guillot X, Sélambarom J et al (2020) Methotrexate an old drug with new tricks. J Mol Sci 20:5023
Casadevall A (2018) Antibody-based vaccine strategies against intracellular pathogens. Curr Opin Immunol 53:74–80
Webster NR, Galley HF (2009) Immunomodulation in the critically ill. Br J Anaesth 103(1):70–81
National Research Council [US] Committee on New Directions in the Study of Antimicrobial Therapeutics: New Classes of Antimicrobials; National Research Council [US] Committee on New Directions in the Study of Antimicrobial Therapeutics: Immunomodulation. Treating Infectious Diseases in a Microbial World: Report of Two Workshops on Novel Antimicrobial Therapeutics. Washington, DC: National Academies Press [US]
Rizk JG, Kalantar-Zadeh K, Mehra MR et al (2020) Pharmaco-immunomodulatory therapy in COVID-19. Drugs 21:1–26
Masihi KN (2000) Immunomodulators in infectious diseases: panoply of possibilites. IntImmunopharmacol 22(12):1083–1091
Lee SJ, Chinen J, Kavanaugh A (2010) Immunomodulator therapy: monoclonal antibodies, fusion proteins, cytokines, and immunoglobulins. J Allergy Clin Immunol 125(2 Suppl 2):S314–S323
Pelfrene E, Mura M, Sanches AC et al (2019) Monoclonal antibodies as anti-infective products: a promising future? Clin Microbiol Infect 25(1):60–64
Fenton C, Scott LJ, Plosker GL (2004) Palivizumab. Paediatr Drugs 6(3):177–197
Mejías A, Chávez-Bueno S, Ríos AM, et al. Anti-respiratory syncytial virus [RSV] neutralizing antibody decreases lung inflammation, airway obstruction, and airway hyperresponsiveness in a murine RSV model. Antimicrob Agents Chemother. 2004;48[5]:1811-
Jacob SA, Iacob DG (2017) Ibalizumab targeting CD4 receptors, an emerging molecule in HIV therapy. Front microbiol 8:2323
Subramanian GM, Cronin PW, Poley G et al (2005) A phase 1 study of PAmAb, a fully human monoclonal antibody against Bacillus anthracis protective antigen, in healthy volunteers. Clin Infect Dis 41:12–20
U.S. Food and Drug Administration. Coronavirus COVID-19 Update FDA Authorizes Monoclonal Antibodies Treatment COVID-19]. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fdaauthorizes-monoclonal-antibodies-treatment-covid-19
Roberts A, Thomas WD, Guarner J et al (2006) Therapy with a severe acute respiratory syndrome-associated coronavirus-neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J Infect Dis 193:685–692
Pachl J, Svoboda P, Jacobs F et al (2006) A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis 42:1404–1413
Matthews RC, Burnie JP (2005) Human recombinant antibody to HSP90: a natural partner in combination therapy. Curr Mol Med 5(4):403–411
Bootz F, Neri D (2016) Immunocytokines: a novel class of products for the treatment of chronic inflammation and autoimmune conditions. Drug Discov Today 21:180–218
Lange CM, Jacobson IM, Rice CM, Zeuzem S (2014) Emerging therapies for the treatment of hepatitis C. EMBO Mol Med 6:4
Ferreira VL, Borba HH, Bonetti AF, et al. Cytokines and Interferons: Types and Functions, Autoantibodies and Cytokines. Wahid Ali Khan: Intech Open. 2018
Chang J, Guo JT (2015) Treatment of chronic hepatitis B with pattern recognition receptor agonists: Current status and potential for a cure. Antiviral Res 121:152–159
Manishi J (1994) Interferon-alpha, beta, gamma. Gan to kagakuryoho. Cancer Chemother 21:2853
Masihi KN, Schäfer H (2011) Overview of biologic response modifiers in infectious disease. Infect Dis Clin 25(4):723–731
Friedman RM, Contente S (2010) Treatment of hepatitis C infections with interferon: a historical perspective. Hepat Res Treat. 2010:323–326
Strannegård Ö (1999) Interferons and their therapeutic applications. EJIFCC 11:52
Nakane A, Minagawa T, Yasuda I et al (1988) Prevention by gamma interferon of fatal infection with Listeria monocytogenes infection. Infect Immun 56:2011–2015
Leiby DA, Fortier AH, Crawford RM et al (1992) In vivo modulation of the murine immune response to Francisellatularensis LVS by administration of anticytokine antibodies. Infect Immun 60:84–89
Subauste CS, Remington JS (1991) Role of gamma interferon in Toxoplasma gondii infection. Eur J Clin Microbiol Infect Dis 10(2):58–67
Beck JM, Brunette EN, Fuchs HJ et al (1991) Reduction in intensity of Pneumocystis carinii pneumonia in mice by aerosol administration of gamma interferon. Infect Immun 59:3859–3862
Kumaratilake LM, Ferrante A, Rzepczyk C (1991) The role of T lymphocytes in immunity to Plasmodium falciparum. Enhancement of neutrophil-mediated parasite killing by lymhotoxin and IFN-gamma: comparisons with tumor necrosis factor effects. J Immunol 146:762–767
Williams DM, Byrne GI, Grubbs B et al (1988) Role in vivo for gamma interferon in control of pneumonia caused by Clamydia trachomatis. Infect Immun 56:3004–3006
Li H, Jerrells TR, Spitalny GL et al (1987) Gamma interferon as a crucial host defense against Rikettsiaconorii in vivo. Infect Immun 55:1252–1255
Cano LE, Kashino SS, Arruda C et al (1998) Protective role of gamma interferon in experimental pulmonary paracoccidioidomycosis. Infect Immun 66:800–806
Way SS, Borczuk AC, Dominitz R et al (1998) An essential role for gamma interferon in innate resistance to Shigellaflexneri infection. Infect Immun 66:1342–1348
Meng Z, Chen Y, Lu M (2020) Advances in targeting the innate and adaptive immune systems to cure chronic hepatitis B virus infection. Front Immunol 10:3127
Brocker C, Thompson D, Matsumoto A et al (2010) Evolutionary divergence and functions of the human interleukin [IL] gene family. Hum Genomics 5(1):1–26
Oppmann B, Lesley R, Blom B et al (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13(5):715–725
Montaner LJ, Bailer RT, Gordon S (1997) IL-13 acts on macrophages to block the completion of reverse transcription, inhibit virus production, and reduce virus infectivity. J Leukoc Biol 62(1):126–132
Montaner LJ, Doyle AG, Collin M et al (1993) Interleukin 13 inhibits human immunodeficiency virus type 1 production in primary blood-derived human macrophages in vitro. J Exp Med 178:743–747
Lester SN, Li K (2014) Toll-like receptors in antiviral innate immunity. J Mol Biol 426(6):1246–1264
Kumar H, Kawai T, Akira S (2011) Pathogen recognition by the innate immune system. Int Rev Immunol 30(1):16–34
Hennessy EJ, Parker AE, O'neill LA. Targeting Toll-like receptors: emerging therapeutics? Nat Rev Drug Discov. 2010; 9[4]:293–307
Rozy A, Chorostowska-Wynimko J (2008) Bacterial immunostimulants — mechanism of action and clinical application in respiratory diseases. Pneumonol Alergol Pol 76:353–359
Ruah SB, Ruah C, van Aubel A, Abel S, Elsasser U (2001) Efficacy of a polyvalent bacterial lysate in children with recurrent respiratory tract infections. Adv Ther 18:151–162
Kasturi SP, Skountzou I, Albrecht RA et al (2011) Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547
Carty M, Bowie AG (2010) Recent insights into the role of Toll-like receptors in viral infection. Clin Exp Immunol 161(3):397–406
Cannon MJ, Stott EJ, Taylor G et al (1987) Clearance of persistent respiratory syncytial virus infections immune deficient mice following transfer primed. T cells Immunology 62(1):133–138
Wykes MN, Lewin SR (2018) Immune checkpoint blockade in infectious diseases. Nat Rev Immunol 18(2):91–104. https://doi.org/10.1038/nri.2017.112
Singh BP, Vij S, Hati S (2014) Functional significance of bioactive peptides derived from soybean. Peptides 54:171–179
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Lei J, Sun L, Huang S et al (2019) The antimicrobial peptides and their potential clinical applications. Am J Transl Res 11:3919
Arnett E, Seveau S (2011) The multifaceted activities of mammalian defensins. Curr Pharm Des 17:4254–4426
Hilchie AL et al (2013) Immune modulation by multifaceted cationic host defense [antimicrobial] peptides. Nat Chem Biol 9:761–768
Harder J, Bartels J, Christophers E et al (2001) Isolation and characterization of human β-defensin-3, a novel human inducible peptide antibiotic. J BiolChem 276:5707–5713
Ganz T, Selsted ME, Szklarek D et al (1985) Defensins. Natural peptide antibiotics of human neutrophils. J Clin Investig 76:1427–1435
Daher KA, Selsted ME, Lehrer RI (1986) Direct inactivation of viruses by human granulocyte defensins. J Virol 60:1068–1074
Soruri A, Grigat J, Forssmann U et al (2007) β-Defensins chemoattract macrophages and mast cells but not lymphocytes and dendritic cells. Eur J Immunol 37:2474–2486
Yang D, Chertov O, Bykovskaia SN et al (1999) β-Defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 286:525–528
Chertov O, Michiel DF, Xu L et al (1996) Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8- stimulated neutrophils. J Biol Chem 271:2935–2940
Holly MK, Diaz K, Smith JG (2017) Defensins in viral infection and pathogenesis. Annu Rev Virol 4:369–391
Chessa C, Bodet C, Jousselin C et al (2020) Antiviral and immunomodulatory properties of antimicrobial peptides produced by human keratinocytes. Fron microbiol 11:1155
Sørensen OE, Follin P, Johnsen AH et al (2001) Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood 97(12):3951–3959
Rivas-Santiago B, Castañeda-Delgado JE, Rivas Santiago CE, et al. Ability of innate defence regulator peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosis infections in animal models. PloS one. 2013;8[3]:e59119.
SteinstraesserL, Hirsch T, Schulte M, et al. Innate defense regulator peptide 1018 in wound healing and wound infection. PLoS One. 2012;7[8]:e39373
Bolouri H, Sävman K, Wang W et al (2014) Innate defense regulator peptide 1018 protects against perinatal brain injury. Ann Neurol 75(3):395–410
Shestakov A, Jenssen H, Hancock RE et al (2013) Synthetic analogues of bovine bactenecindodecapeptide reduces herpes simplex virus type 2 infectivity in mice. Antiviral Res 100(2):455–459
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–778
A specific peptide with immunomodulatory activity from Pseudostellaria heterophylla and the action mechanism
Cai J, Li X, Du H, Jiang C et al (2020) Immunomodulatory significance of natural peptides in mammalians: Promising agents for medical application. Immunobiology 225:151936
Ahmed A, Siman-Tov G, Hall G et al (2019) Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses 11:704. https://doi.org/10.3390/v11080704
Paucek RD, Baltimore D, Li G (2019) The cellular immunotherapy revolution: arming the immune system for precision therapy. Trends Immunol 40(4):292–309
Saha K, Wong PK (1992) Protective role of cytotoxic lymphocytes against murine leukaemia virus-induced neurologic disease and immunodeficiency is enhanced by the presence of helper T cells. Virol 188(2):921–925
Sautto GA, Wisskirchen K, Clementi N et al (2016) Chimeric antigen receptor [CAR]-engineered T cells redirected against hepatitis C virus [HCV] E2 glycoprotein. Gut 65:512–523
Proff J, Brey CU, Ensser A et al (2018) Turning the tables on cytomegalovirus: targeting viral Fc receptors by CARs containing mutated CH2–CH3 IgG spacer domains. J Transl Med 16(1):1–2
Carrillo MA, Zhen A, Zack JA et al (2017) New approaches for the enhancement of chimeric antigen receptors for the treatment of HIV. Transl Res 187:83–92
Olbrich H, Theobald SJ, Slabik C et al (2020) Adult and cord blood-derived high-affinity gB-CAR-T cells effectively react against human Cytomegalovirus infections. Hum Gene Ther 31(7–8):423–439
Lamontagne L, Jolicoeur P, Decarie D et al (1996) Effect of adoptive transfer of CD4, CD8 and B cells on recovery from MHV3-induced immune deficiencies. Immunology 88(20):220–229
Parida SK, Poiret T, Zhenjiang L, et al... T-cell therapy: options for infectious diseases. Arch Clin Infect Dis. 2015;61[suppl_3]:S217–24
Hegde NR, Rao PP, Bayry J et al (2009) Immunotherapy of viral infections. Immunotherapy 1(4):691–711
Masson F, Mount AM, Wilson NS et al (2008) Dendritic cells: driving the differentiation programme of T cells in viral infections. Immunol Cell Biol 86(4):333–342
Lyse Darwish, Samira Mubareka & W Conrad Liles Immunomodulatory therapy for severe influenza. Expert Rev Anti Infect Ther. 2011:7, 807–822
Khoury M, Cuenca J, Cruz FF et al (2020) Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. https://doi.org/10.1183/13993003.00858-2020
Sleem A, Saleh F (2020) Mesenchymal stem cells in the fight against viruses: Face to face with the invisible enemy. Curr Res Transl Med 68(3):105–110
Zhen A, Peterson CW, Carrillo MA, et al. Correction: Long-term persistence and function of hematopoietic stem cell-derived chimeric antigen receptor T cells in a nonhuman primate model of HIV/AIDS. PLoS Pathog. 2018;14[3]:e1006891.
Acknowledgements
Authors wish to express their thanks to the Director and Head, Biomedical Technology Wing, Sree Chitra Tirunal Institute for Medical Sciences and Technology [Govt. of India], Trivandrum, Kerala, India for their support and providing the infrastructure to carry out this work.
Funding
There is no funding from outside agencies. The infrastructure support is provided by the parent Institute.
Author information
Authors and Affiliations
Contributions
Sangetha: Design, draft, literature search, data collection, data analysis, interpretation. Joseph: data collection, data analysis. Mohanan: Design, data analysis, interpretation, final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Consent or publication
All the authors agreed to submit the manuscript in the journal. The same has approved by the parent Institute.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sangeetha Vijayan, P., Xavier, J. & Valappil, M.P. A review of immune modulators and immunotherapy in infectious diseases. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04825-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04825-w