Abstract
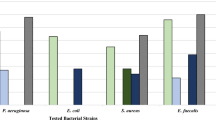
The purpose of this study was to determine whether or not there were significant differences in the antibacterial potential of Thuja occidentalis collected from four distinct geographical sites, namely Chamba (Himachal Pradesh, India), Jalandhar (Punjab, India), Aurangabad (Bihar, India) and Kakching (Manipur, India). The plant extracts were prepared in three different solvents: ethanol, methanol, and acetone. The antibacterial potential of the plant extracts was tested against five different bacterial species using well diffusion test. The minimum inhibitory and bactericidal concentrations of the plant sample exhibiting maximum zone of inhibition against different bacterial strains were calculated. Further, the total phenols, flavonoids, and antioxidant efficacy (using DPPH assay) were also analysed biochemically. The activity of different antioxidant enzymes including SOD, CAT and APX were also recorded as these enzymes protect the cells from free radical damage. GC–MS analysis was also performed on all plant extracts to identify the bioactive components. The results showed that the T. occidentalis collected from the Kakching, Manipur, East side of India showed the highest zone of inhibition against all the bacterial strains, followed by Chamba, Jalandhar, and lastly Aurangabad. To analyse the impact of phytochemicals on the antibacterial efficacy, a correlation was drawn between the biochemical parameters and zone of inhibition using Karl Pearson’s method. Most bacterial species demonstrated a positive correlation between antibacterial effectiveness (zone of inhibition) and biochemical markers. The GC–MS study revealed positive correlation between zone of inhibition and peak area percentages of α-Pinene, β-caryophyllene, Germacrene-D, and Humulene in all bacterial species indicating that these chemicals may play a key role in the bactericidal potential of T. occidentalis. Based on the results of this investigation, it is evident that the antibacterial effectiveness of T. occidentalis varies with its geographical location which may be attributed to the differences in the phytochemical makeup.





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this manuscript.
References
Builders PF (2018) Introduction to herbal medicine. IntechOpen, London, pp 1–9
Mirghafourvand M, Mohammad-Alizadeh-Charandabi S, Ahmadpour P, Javadzadeh Y (2016) Effects of Vitex agnus and Flaxseed on cyclic mastalgia: a randomized controlled trial. Complement Ther Med 24:90–95. https://doi.org/10.1016/j.ctim.2015.12.009
Saroya AS (2011) Herbalism, phytochemistry, and ethnopharmacology. CRC Press, Science Publishers, Boca Raton
Ekor M (2014) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 4:177. https://doi.org/10.3389/fphar.2013.00177
Institute of Medicine (2005) Complementary and alternative medicine in the United States. National Academies Press, Washington, D.C.
Calapai G (2008) European legislation on herbal medicines: a look into the future. Drug Saf 31:428–431. https://doi.org/10.2165/00002018-200831050-00009
Silva IS, Nicolau LAD, Sousa FBM et al (2017) Evaluation of anti-inflammatory potential of aqueous extract and polysaccharide fraction of T. occidentalis Linn. in mice. Int J Biol Macromol 105:1105–1116. https://doi.org/10.1016/j.ijbiomac.2017.07.142
Chang LC, Song LL, Park EJ et al (2000) Bioactive Constituents of T. occidentalis. J Nat Prod 63:1235–1238. https://doi.org/10.1021/np0001575
IBP (2023) In: India Biodiversity Portal. https://indiabiodiversity.org/page/show/4246005. Accessed 15 Jan 2023.
Farrar JL (1996) Les arbres du Canada. Saint-Laurent, Quebec (Canada) FIDES/Service Canadien des Forets/Groupe Cummunication Canada.
Thuja occidentalis L. (THUJA_OCC). https://www.upov.int/genie/details.xhtml?cropId=5558. Accessed 1 Apr 2023.
Millspaugh CF (1974) American medicinal plants: an illustrated and descriptive guide to plants indigenous to and naturalized in the United States which are used in medicine. Dover Publications, New York
Naser B, Bodinet C, Tegtmeier M, Lindequist U (2005) T. occidentalis (Arbor vitae): a review of its pharmaceutical, pharmacological and clinical properties. Evid Based Complement Altern Med 2:69–78. https://doi.org/10.1093/ecam/neh065
KüpeliAkkol E, İlhan M, Ayşe Demirel M et al (2015) T. occidentalis L. and its active compound, α-thujone: Promising effects in the treatment of polycystic ovary syndrome without inducing osteoporosis. J Ethnopharmacol 168:25–30. https://doi.org/10.1016/j.jep.2015.03.029
Reitz HD, Hergarten H (1990) ImmunmodulatorenmitpflanzlichenWirkstoffen–2. Teil: einewissenschaftlicheStudie am BeispielEsberitox® N. Notabene Med 20:304–306
Shimada K (1956) Contribution to anatomy of the central nervous system of the Japanese. XI. Upon the vermal arbor vitae. Okajimas Folia Anat Jpn 28:207–227. https://doi.org/10.2535/ofaj1936.28.1-6_207
Biswas R, Mandal SK, Dutta S et al (2011) Thujone-rich fraction of T. occidentalis demonstrates major anti-cancer potentials: evidences from in vitro studies on A375 Cells. Evid Based Complement Altern Med. https://doi.org/10.1093/ecam/neq042
Caruntu S, Ciceu A, Olah NK et al (2020) T. occidentalis L. (Cupressaceae): ethnobotany, phytochemistry and biological activity. Molecules 25:5416. https://doi.org/10.3390/molecules25225416
Beuscher N, Kopanski L (1986) Purification and Biological Characterization of Antiviral Substances from T. occidentalis. Planta Med 52:555–556. https://doi.org/10.1055/s-2007-969369
Cummings S, Ullman D (1994) Everybody’s guide to homeopathic medicines, Rev. and expanded. J.P. Tarcher ; Distributed by St. Martin’s Press, Los Angeles, New York.
Hochvaldová L, Panáček D, Válková L et al (2022) Restoration of antibacterial activity of inactive antibiotics via combined treatment with a cyanographene/Ag nanohybrid. Sci Rep 12:5222. https://doi.org/10.1038/s41598-022-09294-7
Kumar S, Yadav M, Yadav A, Yadav JP (2017) Impact of spatial and climatic conditions on phytochemical diversity and in vitro antioxidant activity of Indian Aloe vera (L.) Burm.f. S Afr J Bot 111:50–59. https://doi.org/10.1016/j.sajb.2017.03.012
Ganskopp D, Bohnert D, Bohnert D, Bohnert D (2003) Mineral concentration dynamics among 7 Northern Great Basin grasses. J Range Manag 56:174. https://doi.org/10.2307/4003902
Leber AL (2016) Preparation of routine media and reagents used in antimicrobial susceptibility testing. In: Clinical microbiology procedures handbook. ASM Press, Washington, DC, USA, p 5.20.1.1–5.20.3.10.
Thakur M, Kaur T (2022) Impact of geographical location on the antibacterial activity of Cuscutareflexa. J Indian bot Soc 102:164–170. https://doi.org/10.5958/2455-7218.2022.00005.5
Felhi S, Daoud A, Hajlaoui H et al (2017) Solvent extraction effects on phytochemical constituents profiles, antioxidant and antimicrobial activities and functional group analysis of Ecballium elaterium seeds and peels fruits. Food Sci Technol 37:483–492. https://doi.org/10.1590/1678-457x.23516
Adeyemi AI, Vincent OI, Olujenyo OM (2018) Phytochemical screening and antifungal activity of Chromolaena odorata extracts against isolate of Phytophthora megakarya using agar-well diffusion method. Asian J Med Biol Res 4:7–13. https://doi.org/10.3329/ajmbr.v4i1.36815
Andrews JM (2001) Determination of minimum inhibitory concentrations. J Antimicrob Chemother 48:5–16. https://doi.org/10.1093/jac/48.suppl_1.5
Parvekar P, Palaskar J, Metgud S et al (2020) The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater Invest Dent 7:105–109. https://doi.org/10.1080/26415275.2020.1796674
Makkar HPS, Blümmel M, Borowy NK, Becker K (1993) Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agric 61:161–165. https://doi.org/10.1002/jsfa.2740610205
Sharma P, Kumar V, Khosla R, Guleria P (2020) Exogenous naringenin improved digestible protein accumulation and altered morphology via VrPIN and auxin redistribution in Vigna radiata. 3 Biotech 10:431. https://doi.org/10.1007/s13205-020-02428-6
Chang C-C, Yang M-H, Wen H-M, Chern J-C (2002) Estimation of total flavonoid content in propolis by two complementary colometric methods. J Food Drug Anal 10:3. https://doi.org/10.38212/2224-6614.2748
Guleria P, Yadav SK (2014) Overexpression of a glycosyltransferase gene SrUGT74G1 from Stevia improved growth and yield of transgenic Arabidopsis by catechin accumulation. Mol Biol Rep 41:1741–1752. https://doi.org/10.1007/s11033-014-3023-y
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occur Higher Plants Plant Physiol 59:309–314. https://doi.org/10.1104/pp.59.2.309
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Blyth S (1994) Karl Pearson and the correlation curve. Int Stat Rev 62:393. https://doi.org/10.2307/1403769
Dubey A, Raja W, Bairagi Y (2017) Evaluation of antimicrobial activity of T. occidentalis extract against some human pathogenic bacteria. WJPLS 3:97–103
Seo K-S, Jin SW, Choi S, Yun KW (2017) Antibacterial Activity of T. occidentalis, Thuja orientalis and Chamaecyparis obtusa. IJPQA. https://doi.org/10.25258/ijpqa.v8i03.9567 .
TekadayD AR, Jain S (2020) Antimicrobial, antioxidant and phytochemical investigation of T. occidentalis (Arbor vitae) leave extract. GSC Biol and Pharm Sci 12:108–116. https://doi.org/10.30574/gscbps.2020.12.3.0292
Bellili S, Aouadhi C, Dhifi W, et al (2018) The influence of organs on biochemical properties of tunisian T. occidentalis Essential Oils. Symmetry 10:649. https://doi.org/10.3390/sym10110649.
Jahan N, Ahmad M, MehjabeenZia-ul-haq M, Alam SM, Qureshi M (2010) Antimicrobial screening of some medicinal plants of Pakistan. Pak J Bot 42:4281–4284
Nnamonu EI (2022) Evaluation of antimicrobial and bioactive potentials of T. occidentalis on microorganisms. J Adv Res Appl Sci 8:20–27. https://doi.org/10.53555/nnas.v8i1.1272
Rice-evans CA, Miller NJ, Bolwell PG et al (1995) The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radical Res 22:375–383. https://doi.org/10.3109/10715769509145649
Yogesh K, Ali J (2014) Antioxidant potential of thuja (T. occidentalis) cones and peach (Prunus persia) seeds in raw chicken ground meat during refrigerated (4 ± 1 °C) storage. J Food Sci Technol 51:1547–1553. https://doi.org/10.1007/s13197-012-0672-5
Mahboubi A, Asgarpanah J, Sadaghiyani PN, Faizi M (2015) Total phenolic and flavonoid content and antibacterial activity of Punica granatum L. var pleniflora flowers (Golnar) against bacterial strains causing foodborne diseases. BMC Complement Altern Med 15:366. https://doi.org/10.1186/s12906-015-0887-x
Baba SA, Malik SA (2014) Evaluation of antioxidant and antibacterial activity of methanolic extracts of Gentiana kurrooroyle. Saudi J Biol Sci 21:493–498. https://doi.org/10.1016/j.sjbs.2014.06.004
Dubey S, Batra A (2009) Role of phenolics in anti-atherosclerotic property of T. occidentalis Linn. Ethnobot Leaflets 13:791–800
Lü J-M, Lin PH, Yao Q, Chen C (2010) Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med 14:840–860. https://doi.org/10.1111/j.1582-4934.2009.00897.x
Borges MFDA, Lacerda RDS, Correia JPDA, et al (2022) Potential Antibacterial Action of α-Pinene. In: The 2nd International Electronic Conference on Antibiotics & mdash; Drugs for Superbugs: Antibiotic Discovery, Modes of Action And Mechanisms of Resistance. MDPI, p 11. https://doi.org/10.3390/eca2022-12709.
Dahham S, Tabana Y, Iqbal M et al (2015) The anticancer, antioxidant and antimicrobial properties of the sesquiterpene β-caryophyllene from the essential oil of Aquilaria crassna. Molecules 20:11808–11829. https://doi.org/10.3390/molecules200711808
Pérez Zamora C, Torres C, Nuñez M (2018) Antimicrobial activity and chemical composition of essential oils from verbenaceae species growing in South America. Molecules 23:544. https://doi.org/10.3390/molecules23030544
Rossato TCDA, Alves T, Cuevas-Suárez CE et al (2022) Effect of alpha-humulene incorporation on the properties of experimental light-cured periodontal dressings. Braz Oral Res 36:e091. https://doi.org/10.1590/1807-3107bor-2022.vol36.0091
Jang HI, Rhee KJ, Eom YB (2020) Antibacterial and antibiofilm effects of α-humulene against Bacteroides fragilis. Can J Microbiol 66:389–399. https://doi.org/10.1139/cjm-2020-0004
Mitić ZS, Jovanović B, Jovanović SČ et al (2019) Essential oils of Pinus halepensis and P. heldreichii: chemical composition, antimicrobial and insect larvicidal activity. Ind Crops Products 140:111702. https://doi.org/10.1016/j.indcrop.2019.111702
Ceyhan-Güvensen N, Keskin D (2016) Chemical content and antimicrobial properties of three different extracts of Mentha pulegium leaves from Mugla Region, Turkey. J Environ Biol 37:1341–1346
ICZ (2020) India Climate Zone, Weather By Month and Historical Data. https://tcktcktck.org/india. Accessed 15 Jan 2020.
Acknowledgements
We would like to thank DAV University for providing the necessary infrastructure for the present research work. The authors would also like to thank Dr. Sapna Sethi, Associate Professor, Department of Chemistry, DAV University, Jalandhar for helping in analysis of GC–MS results.
Funding
The authors declare that no funds, grants, or other support were received for conducting the research work.
Author information
Authors and Affiliations
Contributions
Mr. Manish Thakur prepared the material, collected the data, carried out the analysis, and wrote the manuscript. Ms. Ayushi Gautam carried out the biochemical analysis. The work was supervized by Dr. Tejinder Kaur and Dr. Praveen Guleria. The manuscript was edited by Dr. Tejinder Kaur and Dr. Ranbir Chander Sobti.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
The authors give consent to Molecular and Cellular Biochemistry journal to publish the present research work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thakur, M., Guleria, P., Sobti, R.C. et al. Comparative analysis of the antibacterial efficacy and bioactive components of Thuja occidentalis obtained from four different geographical sites. Mol Cell Biochem 479, 283–296 (2024). https://doi.org/10.1007/s11010-023-04729-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04729-9