Abstract
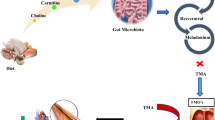
Probiotics are considered to represent important modulators of gastrointestinal health through increased colonization of beneficial bacteria thus altering the gut microflora. Although these beneficial effects of probiotics are now widely recognized, emerging evidence suggests that alterations in the gut microflora also affect numerous other organ systems including the heart through a process generally referred to as the gut-heart axis. Moreover, cardiac dysfunction such as that seen in heart failure can produce an imbalance in the gut flora, known as dysbiosis, thereby further contributing to cardiac remodelling and dysfunction. The latter occurs by the production of gut-derived pro-inflammatory and pro-remodelling factors which exacerbate cardiac pathology. One of the key contributors to gut-dependent cardiac pathology is trimethylamine N-oxide (TMAO), a choline and carnitine metabolic by-product first synthesized as trimethylamine which is then converted into TMAO by a hepatic flavin-containing monooxygenase. The production of TMAO is particularly evident with regular western diets containing high amounts of both choline and carnitine. Dietary probiotics have been shown to reduce myocardial remodelling and heart failure in animal models although the precise mechanisms for these effects are not completely understood. A large number of probiotics have been shown to possess a reduced capacity to synthesize gut-derived trimethylamine and therefore TMAO thereby suggesting that inhibition of TMAO is a factor mediating the beneficial cardiac effects of probiotics. However, other potential mechanisms may also be important contributing factors. Here, we discuss the potential benefit of probiotics as effective therapeutic tools for attenuating myocardial remodelling and heart failure.




Similar content being viewed by others
Data availability
Not applicable.
References
Cleland JGF, van Veldhuisen DJ, Ponikowski P (2019) The year in cardiology 2018: heart failure. Eur Heart J 40:651–661. https://doi.org/10.1093/eurheartj/ehz010
Inamdar AA, Inamdar AC (2016) Heart failure: Diagnosis, management and utilization. J Clin Med 5:62. https://doi.org/10.3390/jcm5070062
Kim DH, Chien F-J, Eisen HJ (2017) Pharmacologic management for heart failure and emerging therapies. Curr Cardiol Rep 19:94. https://doi.org/10.1007/s11886-017-0899-x
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW (2022) 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of cardiology/american heart association joint committee on clinical practice guidelines. Circulation 145:e876–e894. https://doi.org/10.1161/CIR.0000000000001062
Ziaeian B, Fonarow GC (2016) Epidemiology and aetiology of heart failure. Nat Rev Cardiol 13:368–378. https://doi.org/10.1038/nrcardio.2016.25
Tomasoni D, Fonarow GC, Adamo M, Anker SD, Butler J, Coats AJS, Filippatos G, Greene SJ, McDonagh TA, Ponikowski P, Rosano G, Seferovic P, Vaduganathan M, Voors AA, Metra M (2022) Sodium-glucose co-transporter 2 inhibitors as an early, first-line therapy in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 24:431–441. https://doi.org/10.1002/ejhf.2397
Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC (2017) Heart Failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol 70:2476–2486. https://doi.org/10.1016/j.jacc.2017.08.074
Lancet T (2018) Heart failure: the need for improved treatment and care. The Lancet 392:451. https://doi.org/10.1016/S0140-6736(18)31737-9
Rodrigues PG, Leite-Moreira AF, Falcão-Pires I (2016) Myocardial reverse remodeling: how far can we rewind? Am J Physiol Heart Circ Physiol 310:H1402–H1422. https://doi.org/10.1152/ajpheart.00696.2015
Bhatt AS, Ambrosy AP, Velazquez EJ (2017) Adverse remodeling and reverse remodeling after myocardial infarction. Curr Cardiol Rep 19:71. https://doi.org/10.1007/s11886-017-0876-4
Karmazyn M, Gan XT (2017) Treatment of the cardiac hypertrophic response and heart failure with ginseng, ginsenosides, and ginseng-related products. Can J Physiol Pharmacol 95:1170–1176. https://doi.org/10.1139/cjpp-2017-0092
Mashour NH, Lin GI, Frishman WH (1998) Herbal medicine for the treatment of cardiovascular disease: clinical considerations. Arch Int Med 158:2225–2234. https://doi.org/10.1001/archinte.158.20.2225
Puebla-Barragan S, Reid G (2019) Forty-five-year evolution of probiotic therapy. Microb Cell 6:184–196. https://doi.org/10.15698/mic2019.04.673
Mackowiak PA (2013) Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health 1:52. https://doi.org/10.3389/fpubh.2013.00052
Krawczyk RT, Banaszkiewicz A (2021) Dr. Józef Brudziński - the true “Father of probiotics.” Benef Microbes 12:211–213. https://doi.org/10.3920/BM2020.0201
Ozen M (2015) Dinleyici EC (2015) The history of probiotics: the untold story. Benef Microbes 6:159–165. https://doi.org/10.3920/BM2014.0103
Gasbarrini G, Bonvicini F, Gramenzi A (2016) Probiotics History. J Clin Gastroenterol 50 Suppl 2. Proceedings from the 8th Probiotics, Prebiotics & New Foods for Microbiota and Human Health meeting held in Rome, Italy on September 13–15, 2015:S116-S119. https://doi.org/10.1097/MCG.0000000000000697
Santacroce L, Charitos IA, Bottalico L (2019) A successful history: probiotics and their potential as antimicrobials. Expert Rev Anti Infect Ther 17:635–645. https://doi.org/10.1080/14787210.2019.1645597
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514. https://doi.org/10.1038/nrgastro.2014.66
Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev 17:259–275. https://doi.org/10.1079/NRR200479
Valcheva R, Dieleman LA (2016) Prebiotics: Definition and protective mechanisms. Best Pract Res Clin Gastroenterol 30:27–37. https://doi.org/10.1016/j.bpg.2016.02.008
Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, Berenjian A, Ghasemi Y (2019) Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 8:92. https://doi.org/10.3390/foods8030092
Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, Sanders ME (2020) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol 17:687–701. https://doi.org/10.1038/s41575-020-0344-2
Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA (2019) Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol 16:605–616. https://doi.org/10.1038/s41575-019-0173-3(Authorcorrection.In:NatRevGastroenterolHepatol(2019)16:642)
Zagórska A, Marcinkowska M, Jamrozik M, Wiśniowska B, Paśko P (2020) From probiotics to psychobiotics - the gut-brain axis in psychiatric disorders. Benef Microbes 11:717–732. https://doi.org/10.3920/BM2020.0063
Gazerani P (2019) Probiotics for Parkinson’s Disease. Int J Mol Sci 20:4121. https://doi.org/10.3390/ijms20174121
Wang L, Guo MJ, Gao Q, Yang JF, Yang L, Pang XL, Xiang XJ (2018) The effects of probiotics on total cholesterol: A meta-analysis of randomized controlled trials. Medicine (Baltimore) 97:e9679. https://doi.org/10.1097/MD.0000000000009679
Gómez-Guzmán M, Toral M, Romero M, Jiménez R, Galindo P, Sánchez M, Zarzuelo MJ, Olivares M, Gálvez J, Duarte J (2015) Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res 59:2326–2336. https://doi.org/10.1002/mnfr.201500290
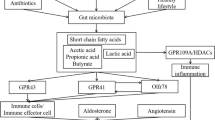
Robles-Vera I, Toral M, de la Visitación N, Sánchez M, Gómez-Guzmán M, Romero M, Yang T, Izquierdo-Garcia JL, Jiménez R, Ruiz-Cabello J, Guerra-Hernández E, Raizada MK, Pérez-Vizcaíno F, Duarte J (2020) Probiotics prevent dysbiosis and the rise in blood pressure in genetic hypertension: Role of short-chain fatty acids. Mol Nutr Food Res 64:e1900616. https://doi.org/10.1002/mnfr.201900616
Kong CY, Li ZM, Mao YQ, Chen HL, Hu W, Han B, Wang LS (2021) Probiotic yogurt blunts the increase of blood pressure in spontaneously hypertensive rats via remodeling of the gut microbiota. Food Funct 12:9773–9783. https://doi.org/10.1039/d1fo01836a
do Nascimento LCP, de Souza EL, de Luna Freire MO, de Andrade Braga V, de Albuqeurque TMR, Lagranha CJ, de Brito Alves JL (2022) Limosilactobacillus fermentum prevents gut-kidney oxidative damage and the rise in blood pressure in male rat offspring exposed to a maternal high-fat diet. J Dev Orig Health Dis 13: 719–726. doi:https://https://doi.org/10.1017/S2040174422000198. Erratum in: J Dev Orig Health Dis (2022) 13: 817. PMID: 35437140
Robles-Vera I, de la Visitación N, Toral M, Sánchez M, Romero M, Gómez-Guzmán M, Yang T, Izquierdo-García JL, Guerra-Hernández E, Ruiz-Cabello J, Raizada MK, Pérez-Vizcaíno F, Jiménez R, Duarte J (2020) Probiotic Bifidobacterium breve prevents DOCA-salt hypertension. FASEB J 34:13626–13640. https://doi.org/10.1096/fj.202001532R
Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, Bryan RM Jr, Durgan DJ (2018) Prebiotics, probiotics, and acetate supplementation prevent hypertension in a model of obstructive sleep apnea. Hypertension 72:1141–1150. https://doi.org/10.1161/HYPERTENSIONAHA.118.11695
Chi C, Li C, Wu D, Buys N, Wang W, Fan H, Sun J (2020) Effects of probiotics on patients with hypertension: a systematic review and meta-analysis. Curr Hypertens Rep 225:34. https://doi.org/10.1007/s11906-020-01042-4
Nagatomo Y, Tang WH (2015) Intersections between microbiome and heart failure: revisiting the gut hypothesis. J Card Fail 21:973–980. https://doi.org/10.1016/j.cardfail.2015.09.017
Kamo T, Akazawa H, Suzuki JI, Komuro I (2017) Novel concept of a heart-gut axis in the pathophysiology of heart failure. Korean Circ J 47:663–669. https://doi.org/10.4070/kcj.2017.0028
Tang WHW, Bäckhed F, Landmesser U, Hazen SL (2019) Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol 73:2089–2105. https://doi.org/10.1016/j.jacc.2019.03.024
Luedde M, Winkler T, Heinsen FA, Rühlemann MC, Spehlmann ME, Bajrovic A, Lieb W, Franke A, Ott SJ, Frey N (2017) Heart failure is associated with depletion of core intestinal microbiota. ESC Heart Fail 4:282–290. https://doi.org/10.1002/ehf2.12155
Li L, Zhong SJ, Hu SY, Cheng B, Qiu H, Hu ZX (2021) Changes of gut microbiome composition and metabolites associated with hypertensive heart failure rats. BMC Microbiol 21:141. https://doi.org/10.1186/s12866-021-02202-5
Mamic P, Chaikijurajai T, Tang WHW (2021) Gut microbiome - A potential mediator of pathogenesis in heart failure and its comorbidities: State-of-the-art review. J Mol Cell Cardiol 152:105–117. https://doi.org/10.1016/j.yjmcc.2020.12.001
Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, Scherbakov N, Cramer L, Rauchhaus M, Grosse-Herrenthey A, Krueger M, von Haehling S, Doehner W, Anker SD, Bauditz J (2014) Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol 64:1092–1102. https://doi.org/10.1016/j.jacc.2014.06.1179
Arutyunov GP, Kostyukevich OI, Serov RA, Rylova NV, Bylova NA (2008) Collagen accumulation and dysfunctional mucosal barrier of the small intestine in patients with chronic heart failure. Int J Cardiol 125:240–245. https://doi.org/10.1016/j.ijcard.2007.11.103
Tang TWH, Chen HC, Chen CY, Yen CYT, Lin CJ, Prajnamitra RP, Chen LL, Ruan SC, Lin JH, Lin PJ, Lu HH, Kuo CW, Chang CM, Hall AD, Vivas EI, Shui JW, Chen P, Hacker TA, Rey FE, Kamp TJ, Hsieh PCH (2019) Loss of gut microbiota alters immune system composition and cripples postinfarction cardiac repair. Circulation 139:647–659. https://doi.org/10.1161/CIRCULATIONAHA.118.035235
Hardin SJ, Singh M, Eyob W, Molnar JC, Homme RP, George AK, Tyagi SC (2019) Diet-induced chronic syndrome, metabolically transformed trimethylamine-N-oxide, and the cardiovascular functions. Rev Cardiovasc Med 20:121–128. https://doi.org/10.31083/j.rcm.2019.03.518
Din AU, Hassan A, Zhu Y, Yin T, Gregersen H, Wang G (2019) Amelioration of TMAO through probiotics and its potential role in atherosclerosis. Appl Microbiol Biotechnol 103:9217–9228. https://doi.org/10.1007/s00253-019-10142-4
Witkowski M, Weeks TL, Hazen SL (2020) Gut microbiota and cardiovascular disease. Circ Res 127:553–570. https://doi.org/10.1161/CIRCRESAHA.120.316242
Vourakis M, Mayer G, Rousseau G (2021) The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int J Mol Sci 22:8074. https://doi.org/10.3390/ijms22158074
Duttaroy AK (2021) Role of gut microbiota and their metabolites on atherosclerosis, hypertension and human blood platelet function: A review. Rev Nutr 13:144. https://doi.org/10.3390/nu13010144
Zeisel SH, Warrier M (2017) Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Ann Rev Nutr 37:157–181. https://doi.org/10.1146/annurev-nutr-071816-064732
Boutagy NE, Neilson AP, Osterberg KL, Smithson AT, Englund TR, Davy BM, Hulver MW, Davy KP (2015) Probiotic supplementation and trimethylamine-N-oxide production following a high-fat diet. Obesity (Silver Spring) 23:2357–2363. https://doi.org/10.1002/oby.21212
Chen K, Zheng X, Feng M, Li D, Zhang H (2017) Gut microbiota-dependent metabolite trimethylamine N-oxide contributes to cardiac dysfunction in western diet-induced obese mice. Front Physiol 8:139. https://doi.org/10.3389/fphys.2017.00139
Deanfield JE, Halcox JP, Rabelink TJ (2007) Endothelial function and dysfunction: testing and clinical relevance. Circulation 115:1285–1295. https://doi.org/10.1161/CIRCULATIONAHA.106.652859
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376. https://doi.org/10.1038/288373a0
Fleming I, Busse R (1999) NO: the primary EDRF. J Mol Cell Cardiol 31:5–14. https://doi.org/10.1006/jmcc.1998.0839
Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, Chen Y (2016) Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun 481:63–70. https://doi.org/10.1016/j.bbrc.2016.11.017
Querio G, Antoniotti S, Geddo F, Levi R, Gallo MP (2022) Trimethylamine N-oxide (TMAO) impairs purinergic induced intracellular calcium increase and nitric oxide release in endothelial cells. Int Journal Mol Sci 23:3982. https://doi.org/10.3390/ijms23073982
Li T, Chen Y, Gua C, Li X (2017) Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front Physiol 8:350. https://doi.org/10.3389/fphys.2017.00350
Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, Ou C, Chen M (2019) Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest 99:346–357. https://doi.org/10.1038/s41374-018-0091-y
Schultz Jel J, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T (2002) TGF-β1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J Clin Invest 109:787–796. https://doi.org/10.1172/JCI14190
Li X, Fan Z, Cui J, Li D, Lu J, Cui X, Xie L, Wu Y, Lin Q, Li Y (2022) Trimethylamine N-oxide in heart failure: a meta-analysis of prognostic value. Front Cardiovasc Med 9:817396. https://doi.org/10.3389/fcvm.2022.817396
Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, Wu Y, Hazen SL (2014) Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 64:1908–1914. https://doi.org/10.1016/j.jacc.2014.02.617
Salzano A, Cassambai S, Yazaki Y, Israr MZ, Bernieh D, Wong M, Suzuki T (2022) The gut axis involvement in heart failure: focus on trimethylamine N-oxide. Cardiol Clin 40:161–169. https://doi.org/10.1016/j.ccl.2021.12.004
Israr MZ, Zhan H, Salzano A, Voors AA, Cleland JG, Anker SD, Metra M, van Veldhuisen DJ, Lang CC, Zannad F, Samani NJ, Ng LL, Suzuki T, BIOSTAT-CHF investigators (2022) Surrogate markers of gut dysfunction are related to heart failure severity and outcome-from the BIOSTAT-CHF consortium. Am Heart J 248:108–119. https://doi.org/10.1016/j.ahj.2022.03.002
Konieczny R, Żurawska-Płaksej E, Kaaz K, Czapor-Irzabek H, Bombała W, Mysiak A, Kuliczkowski W (2022) All-cause mortality and trimethylamine-N-oxide levels in patients with cardiovascular disease. Cardiology 147:443–452. https://doi.org/10.1159/000525972
Zong X, Fan Q, Yang Q, Pan R, Zhuang L, Xi R, Zhang R, Tao R (2022) Trimethyllysine, a trimethylamine N-oxide precursor, predicts the presence, severity, and prognosis of heart failure. Front Cardiovasc Med 9:907997. https://doi.org/10.3389/fcvm.2022.907997
Wang G, Kong B, Shuai W, Fu H, Jiang X, Huang H (2020) 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. J Nutr Biochem 78:108341. https://doi.org/10.1016/j.jnutbio.2020.108341
Organ CL, Otsuka H, Bhushan S, Wang Z, Bradley J, Trivedi R, Polhemus DJ, Tang WH, Wu Y, Hazen SL, Lefer DJ (2016) Choline diet and its gut microbe-derived metabolite, trimethylamine N-oxide, exacerbate pressure overload-induced heart failure. Circ Heart Fail 9:e002314. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002314
Zou D, Li Y, Sun G (2021) Attenuation of circulating trimethylamine N-oxide prevents the progression of cardiac and renal dysfunction in a rat model of chronic cardiorenal syndrome. Front Pharmacol 12:751380. https://doi.org/10.3389/fphar.2021.751380
Yuzefpolskaya M, Bohn B, Javaid A, Mondellini GM, Braghieri L, Pinsino A, Onat D, Cagliostro B, Kim A, Takeda K, Naka Y, Farr M, Sayer GT, Uriel N, Nandakumar R, Mohan S, Colombo PC, Demmer RT (2021) Levels of trimethylamine N-oxide remain elevated long term after left ventricular assist device and heart transplantation and are independent from measures of inflammation and gut dysbiosis. Circ Heart Fail 14:e007909. https://doi.org/10.1161/CIRCHEARTFAILURE.120.007909
Yoshida Y, Shimizu I, Shimada A, Nakahara K, Yanagisawa S, Kubo M, Fukuda S, Ishii C, Yamamoto H, Ishikawa T, Kano K, Aoki J, Katsuumi G, Suda M, Ozaki K, Yoshida Y, Okuda S, Ohta S, Okamoto S, Minokoshi Y, Oda K, Sasaoka T, Abe M, Sakimura K, Kubota Y, Yoshimura N, Kajimura S, Zuriaga M, Walsh K, Soga T, Minamino T (2022) Brown adipose tissue dysfunction promotes heart failure via a trimethylamine N-oxide-dependent mechanism. Sci Rep 12:14883. https://doi.org/10.1038/s41598-022-19245-x
Li X, Sun Y, Zhang X, Wang J (2019) Reductions in gut microbiota-derived metabolite trimethylamine N-oxide in the circulation may ameliorate myocardial infarction-induced heart failure in rats, possibly by inhibiting interleukin-8 secretion. Mol Med Rep 20:779–786. https://doi.org/10.3892/mmr.2019.10297
Massagué J, Wotton D (2000) Transcriptional control by the TGF-β/Smad signaling system. EMBO J 19:1745–1754. https://doi.org/10.1093/emboj/19.8.1745
Huc T, Drapala A, Gawrys M, Konop M, Bielinska K, Zaorska E, Samborowska E, Wyczalkowska-Tomasik A, Pączek L, Dadlez M, Ufnal M (2018) Chronic, low-dose TMAO treatment reduces diastolic dysfunction and heart fibrosis in hypertensive rats. Am J Physiol Heart Circ Physiol 315:H1805–H1820. https://doi.org/10.1152/ajpheart.00536.2018
Marques FZ, Mackay CR, Kaye DM (2018) Beyond gut feelings: how the gut microbiota regulates blood pressure. Nat Rev Cardiol 15:20–32. https://doi.org/10.1038/nrcardio.2017.120
Naqvi S, Asar TO, Kumar V, Al-Abbasi FA, Alhayyani S, Kamal MA, Anwar F (2021) A cross-talk between gut microbiome, salt and hypertension. Biomed Pharmacother 134:111156. https://doi.org/10.1016/j.biopha.2020.111156
Liu G, Cheng J, Zhang T, Shao Y, Chen X, Han L, Zhu R, Wu B (2022) Inhibition of microbiota-dependent trimethylamine N-oxide production ameliorates high salt diet-induced sympathetic excitation and hypertension in rats by attenuating central neuroinflammation and oxidative stress. Front Pharmacol 13:856914. https://doi.org/10.3389/fphar.2022.856914
Qiu L, Yang D, Tao X, Yu J, Xiong H, Wei H (2017) Enterobacter aerogenes ZDY01 attenuates choline-induced trimethylamine N-oxide levels by remodeling gut microbiota in mice. J Microbiol Biotechnol 27:1491–1499. https://doi.org/10.4014/jmb.1703.03039
Qiu L, Tao X, Xiong H, Yu J, Wei H (2018) Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct 9:4299–4309. https://doi.org/10.1039/c8fo00349a
Wang Q, Guo M, Liu Y, Xu M, Shi L, Li X, Zhao J, Zhang H, Wang G, Chen W (2022) Bifidobacterium breve and Bifidobacterium longum attenuate choline-induced plasma trimethylamine N-oxide production by modulating gut microbiota in mice. Nutrients 14:1222. https://doi.org/10.3390/nu14061222
Tungsanga S, Panpetch W, Bhunyakarnjanarat T, Udompornpitak K, Katavetin P, Chancharoenthana W, Chatthanathon P, Somboonna N, Tungsanga K, Tumwasorn S, Leelahavanichkul A (2022) Uremia-induced gut barrier defect in 5/6 nephrectomized mice is worsened by Candida administration through a synergy of uremic toxin, lipopolysaccharide, and (1➔3)-β-D-glucan, but is attenuated by Lacticaseibacillus rhamnosus L34. Int J Mol Sci 23:2511. https://doi.org/10.3390/ijms23052511
Miao L, Du J, Chen Z, Shi D, Qu H (2021) Effects of microbiota-driven therapy on circulating trimethylamine-N-oxide metabolism: A systematic review and meta-analysis. Front Cardiovasc Med 8:710567. https://doi.org/10.3389/fcvm.2021.710567
Koppinger MP, Lopez-Pier MA, Skaria R, Harris PR, Konhilas JP (2020) Lactobacillus reuteri attenuates cardiac injury without lowering cholesterol in low-density lipoprotein receptor-deficient mice fed standard chow. Am J Physiol Heart Circ Physiol 319:H32–H41. https://doi.org/10.1152/ajpheart.00569.2019
Danilo CA, Constantopoulos E, McKee LA, Chen H, Regan JA, Lipovka Y, Lahtinen S, Stenman LK, Nguyen TV, Doyle KP, Slepian MJ, Khalpey ZI, Konhilas JP (2017) Bifidobacterium animalis subsp. lactis 420 mitigates the pathological impact of myocardial infarction in the mouse. Benef Microbes 8:257–269. https://doi.org/10.3920/BM2016.0119
Lam V, Su J, Koprowski S, Hsu A, Tweddell JS, Rafiee P, Gross GJ, Salzman NH, Baker JE (2012) Intestinal microbiota determine severity of myocardial infarction in rats. FASEB J 26:1727–1735. https://doi.org/10.1096/fj.11-197921
Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, Nichols M, Ben-Yehuda O (2016) Relationship between infarct size and outcomes following primary PCI: Patient-level analysis from 10 randomized trials. J Am Coll Cardiol 67:1674–1683. https://doi.org/10.1016/j.jacc.2016.01.069
Selker HP, Udelson JE, Ruthazer R, D’Agostino RB, Nichols M, Ben-Yehuda O, Eitel I, Granger CB, Jenkins P, Maehara A, Patel MR, Ohman EM, Thiele H, Stone GW (2017) Relationship between therapeutic effects on infarct size in acute myocardial infarction and therapeutic effects on 1-year outcomes: A patient-level analysis of randomized clinical trials. Am Heart J 188:18–25. https://doi.org/10.1016/j.ahj.2017.02.028
Cookson TA (2021) Bacterial-induced blood pressure reduction: Mechanisms for the treatment of hypertension via the gut. Front Cardiovasc Med 8:721393. https://doi.org/10.3389/fcvm.2021.721393
Yan D, Sun Y, Zhou X, Si W, Liu J, Li M, Wu M (2022) Regulatory effect of gut microbes on blood pressure. Animal Model Exp Med. https://doi.org/10.1002/ame2.12233
Höcht C, Allo MA, Polizio AH, Morettón MA, Carranza A, Chiappetta DA, Choi MR (2022) New and developing pharmacotherapies for hypertension. Expert Rev Cardiovasc Ther 20:647–666. https://doi.org/10.1080/14779072.2022.2105204
Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, Sidaway JE, Martin G, Gloor GB, Swann JR, Reid G, Karmazyn M (2014) Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the rat. Circ Heart Fail 7:491–499. https://doi.org/10.1161/CIRCHEARTFAILURE.113.000978
Karmazyn M, Gan XT, Rajapurohitam V (2013) The potential contribution of circulating and locally produced leptin to cardiac hypertrophy and failure. Can J Physiol Pharmacol 91:883–888. https://doi.org/10.1139/cjpp-2013-0057
Park M, Sweeney G (2013) Direct effects of adipokines on the heart: focus on adiponectin. Heart Fail Rev 18:631–644. https://doi.org/10.1007/s10741-012-9337-8
Ten Hove M, Chan S, Lygate C, Monfared M, Boehm E, Hulbert K, Watkins H, Clarke K, Neubauer S (2005) Mechanisms of creatine depletion in chronically failing rat heart. J Mol Cell Cardiol 38:309–313. https://doi.org/10.1016/j.yjmcc.2004.11.016
Neubauer S, Remkes H, Spindler M, Horn M, Wiesmann F, Prestle J, Walzel B, Ertl G, Hasenfuss G, Wallimann T (1999) Downregulation of the Na+-creatine cotransporter in failing human myocardium and in experimental heart failure. Circulation 100:1847–1850. https://doi.org/10.1161/01.cir.100.18.1847
Huxtable RJ (1993) Taurine and the heart. Cardiovasc Res 27:1136–1137. https://doi.org/10.1093/cvr/27.6.1136a
Schaffer SW, Jong CJ, Ramila KC, Azuma J (2010) Physiological roles of taurine in heart and muscle. J Biomedical Sci. https://doi.org/10.1186/1423-0127-17-S1-S2
Azuma M, Takahashi K, Fukuda T, Ohyabu Y, Yamamoto I, Kim S, Iwao H, Schaffer SW, Azuma J (2000) Taurine attenuates hypertrophy induced by angiotensin II in cultured neonatal rat cardiac myocytes. Eu J Pharmacol 403:181–188. https://doi.org/10.1016/s0014-2999(00)00483-0
Liu J, Ai Y, Niu X, Shang F, Li Z, Liu H, Li W, Ma W, Chen R, Wei T, Li X, Li X (2020) Taurine protects against cardiac dysfunction induced by pressure overload through SIRT1-p53 activation. Chem Biol Interact 317:108972. https://doi.org/10.1016/j.cbi.2020.108972
Bkaily G, Simon Y, Normand A, Jazzar A, Najibeddine H, Khalil A, Jacques D (2022) Short-Communication: Short-term treatment with taurine prevents the development of cardiac hypertrophy and early death in hereditary cardiomyopathy of the hamster and is sex-dependent. Nutrients 14:3287. https://doi.org/10.3390/nu14163287
Beyranvand MR, Khalafi MK, Roshan VD, Choobineh S, Parsa SA, Piranfar MA (2011) Effect of taurine supplementation on exercise capacity of patients with heart failure. J Cardiol 57:333–337. https://doi.org/10.1016/j.jjcc.2011.01.007
Ettinger G, Burton JP, Gloor GB, Reid G (2017) Lactobacillus rhamnosus GR-1 attenuates induction of hypertrophy in cardiomyocytes but not through secreted protein MSP-1 (p75). PLoS ONE 12:e0168622. https://doi.org/10.1371/journal.pone.0168622
Chiang CJ, Chao YP, Ali A, Day CH, Ho TJ, Wang PN, Lin SC, Padma VV, Kuo WW, Huang CY (2021) Probiotic Escherichia coli Nissle inhibits IL-6 and MAPK-mediated cardiac hypertrophy during STZ-induced diabetes in rats. Benef Microbes 12:283–293. https://doi.org/10.3920/BM2020.0094
Chiang CJ, Tsai BC, Lu TL, Chao YP, Day CH, Ho TJ, Wang PN, Lin SC, Padma VV, Kuo WW, Huang CY (2021) Diabetes-induced cardiomyopathy is ameliorated by heat-killed Lactobacillus reuteri GMNL-263 in diabetic rats via the repression of the toll-like receptor 4 pathway. Eur J Nutr 60:3211–3223. https://doi.org/10.1007/s00394-020-02474-z
Hu Y, Pan Z, Huang Z, Li Y, Han N, Zhuang X, Peng H, Gao Q, Wang Q, Yang Lee BJ, Zhang H, Yang R, Bi Y, Xu ZZ (2022) Gut microbiome-targeted modulations regulate metabolic profiles and alleviate altitude-related cardiac hypertrophy in rats. Microbiol Spectr 10:e0105321. https://doi.org/10.1128/spectrum.01053-21
Dronkers TMG, Ouwehand AC, Rijkers GT (2020) Data on global analysis of clinical trials with probiotics. Data Brief 32:106269. https://doi.org/10.1016/j.dib.2020.106269
Costanza AC, Moscavitch SD, Faria Neto HC, Mesquita ET (2015) Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int J Cardiol 179:348–350. https://doi.org/10.1016/j.ijcard.2014.11.034
Moludi J, Saiedi S, Ebrahimi B, Alizadeh M, Khajebishak Y, Ghadimi SS (2021) Probiotics supplementation on cardiac remodeling following myocardial infarction: a single-center double-blind clinical study. J Cardiovasc Transl Res 14:299–307. https://doi.org/10.1007/s12265-020-10052-1
Awoyemi A, Mayerhofer C, Felix AS, Hov JR, Moscavitch SD, Lappegård KT, Hovland A, Halvorsen S, Halvorsen B, Gregersen I, Svardal A, Berge RK, Hansen SH, Götz A, Holm K, Aukrust P, Åkra S, Seljeflot I, Solheim S, Lorenzo A, Gullestad L, Trøseid M, Broch K (2021) Rifaximin or Saccharomyces boulardii in heart failure with reduced ejection fraction: Results from the randomized GutHeart trial. EBioMedicine 70:103511. https://doi.org/10.1016/j.ebiom.2021.103511
Matin SS, Shidfar F, Naderi N, Amin A, Hosseini-Baharanchi FS, Dehnad A (2022) The effect of synbiotic consumption on serum NTproBNP, hsCRP and blood pressure in patients with chronic heart failure: A randomized, triple-blind, controlled trial. Front Nutr 8:822498. https://doi.org/10.3389/fnut.2021.822498
Funding
Studies cited from the authors’ laboratory were supported by a grant (MOP 62764) from the Canadian Institutes of Health Research. MK held a Canada Research Chair in Experimental Cardiology during the course of those studies.
Author information
Authors and Affiliations
Contributions
MK conceived the writing of this review whereas both authors contributed to the writing of the article and final review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Karmazyn, M., Gan, X.T. Probiotics as potential treatments to reduce myocardial remodelling and heart failure via the gut-heart axis: State-of-the-art review. Mol Cell Biochem 478, 2539–2551 (2023). https://doi.org/10.1007/s11010-023-04683-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04683-6