Abstract
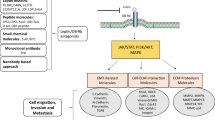
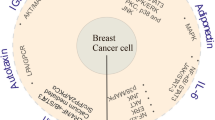
Obesity is a significant risk factor for cancer development. Within the tumor microenvironment, adipocytes interact with cancer cells, immune cells, fibroblasts and endothelial cells, and orchestrate several signaling pathways by secreting bioactive molecules, including adipokines. Adipokines or adipocytokines are produced predominantly by adipocytes and function as autocrine, paracrine and endocrine mediators. Adipokines can exert pro- and anti-inflammatory functions, and they play a pivotal role in the state of chronic low-grade inflammation that characterizes obesity. Epithelial-mesenchymal transition (EMT), a complex biological process whereby epithelial cells acquire the invasive, migratory mesenchymal phenotype is well-known to be implicated in cancer progression and metastasis. Emerging evidence suggests that there is a link between adipokines and EMT. This may contribute to the correlation that has been documented between obesity and cancer progression. This review summarizes the existing body of evidence supporting an association between the process of EMT in cancer and the adipokines leptin, adiponectin, resistin, visfatin/NAMPT, lipocalin-2/NGAL, as well as other newly discovered adipokines including chemerin, nesfatin-1/nucleobindin-2, AZGP1, SFRP5 and FABP4.


Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
Abbreviations
- ADSF:
-
Adipocyte-specific secretory factor
- BMI:
-
Body mass index
- CAP1:
-
Adenylyl cyclase-associated protein-1
- CCAs:
-
Cancer-associated adipocytes
- CCL2:
-
C–C motif chemokine 2
- COX-2:
-
Cyclooxygenase-2
- EMT:
-
Epithelial-mesenchymal transition
- eNAMPT:
-
Extracellular nicotinamide phosphoribosyltransferase
- FABP4:
-
Fatty acid binding protein-4
- FAK:
-
Focal adhesion kinase
- FIZZ3:
-
Protein found in inflammatory zone 3
- HCC:
-
Hepatocellular carcinoma
- iNAMPT:
-
Intracellular Nicotinamide phosphoribosyltransferase
- LCN2:
-
Lipocalin-2
- MCP-1:
-
Monocyte chemotactic protein 1
- MET:
-
Mesenchymal-epithelial transition
- MMPs:
-
Matrix metalloproteinases
- NAMPT:
-
Nicotinamide phosphoribosyltransferase
- NGAL:
-
Neutrophil gelatinase-associated lipocalin
- PAI-1:
-
Plasminogen activator inhibitor-1
- SFRP5:
-
Secreted frizzled-related protein 5
- TADCs:
-
Tumor-associated dendritic cells
- TLR4:
-
Toll-like receptor 4
- TNBC:
-
Triple negative breast cancer
- TNF:
-
Tumor necrosis factor
- ZAG:
-
Zinc-alpha2-glycoprotein
- ZO-1:
-
Zonula occludens-1
References
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working Group (2016) Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med 375(8):794–798. https://doi.org/10.1056/NEJMsr1606602
Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30(7):1073–1081. https://doi.org/10.1093/carcin/bgp127
Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M (2019) Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism 92:121–135. https://doi.org/10.1016/j.metabol.2018.11.001
Mancuso P (2016) The role of adipokines in chronic inflammation. Immunotargets Ther 5:47–56. https://doi.org/10.2147/ITT.S73223
Park J, Morley TS, Kim M, Clegg DJ, Scherer PE (2014) Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 10(8):455–465. https://doi.org/10.1038/nrendo.2014.94
Georgakopoulos-Soares I, Chartoumpekis DV, Kyriazopoulou V, Zaravinos A (2020) EMT factors and metabolic pathways in cancer. Front Oncol 10:499. https://doi.org/10.3389/fonc.2020.00499
Brabletz S, Schuhwerk H, Brabletz T, Stemmler MP (2021) Dynamic EMT: a multi-tool for tumor progression. EMBO J 40(18):e108647. https://doi.org/10.15252/embj.2021108647
Ray A, Cleary MP (2017) The potential role of leptin in tumor invasion and metastasis. Cytokine Growth Factor Rev 38:80–97
Olea-Flores M, Juárez-Cruz JC, Mendoza-Catalán MA, Padilla-Benavides T, Navarro-Tito N (2018) Signaling pathways induced by leptin during epithelial mesenchymal transition in breast cancer. Int J Mol Sci 19(11):3493
Ghasemi A, Saeidi J, Azimi-Nejad M, Hashemy SI (2019) Leptin-induced signaling pathways in cancer cell migration and invasion. Cell Oncol (Dordr) 42(3):243–260. https://doi.org/10.1007/s13402-019-00428-0
Cho BA, Iyengar NM, Zhou XK, Morrow M, Giri DD, Verma A, Elemento O, Pollak M, Dannenberg AJ (2021) Blood biomarkers reflect the effects of obesity and inflammation on the human breast transcriptome. Carcinogenesis 42(10):1281–1292. https://doi.org/10.1093/carcin/bgab066
Bowers LW, Rossi EL, McDonell SB, Doerstling SS, Khatib SA, Lineberger CG, Albright JE, Tang X, deGraffenried LA, Hursting SD (2018) Leptin signaling mediates obesity-associated CSC enrichment and EMT in preclinical TNBC models. Mol Cancer Res 16(5):869–879. https://doi.org/10.1158/1541-7786.MCR-17-0508
Wei X, Liu Y, Gong C, Ji T, Zhou X, Zhang T, Wan D, Xu S, Jin P, Yang X, Li X, Ma D, Yang Z, Gao Q (2017) Targeting leptin as a therapeutic strategy against ovarian cancer peritoneal metastasis. Anticancer Agents Med Chem 17(8):1093–1101. https://doi.org/10.2174/1871520616666161221114454
Wei L, Li K, Pang X, Guo B, Su M, Huang Y, Wang N, Ji F, Zhong C, Yang J, Zhang Z, Jiang Y, Liu Y, Chen T (2016) Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2. J Exp Clin Cancer Res 35(1):166. https://doi.org/10.1186/s13046-016-0446-4
Wang L, Tang C, Cao H, Li K, Pang X, Zhong L, Dang W, Tang H, Huang Y, Wei L, Su M, Chen T (2015) Activation of IL-8 via PI3K/Akt-dependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol Ther 16(8):1220–1230. https://doi.org/10.1080/15384047.2015.1056409
Andò S, Barone I, Giordano C, Bonofiglio D, Catalano S (2014) The Multifaceted mechanism of leptin signaling within tumor microenvironment in driving breast cancer growth and progression. Front Oncol 4:340. https://doi.org/10.3389/fonc.2014.00340
Fujisaki K, Fujimoto H, Sangai T, Nagashima T, Sakakibara M, Shiina N, Kuroda M, Aoyagi Y, Miyazaki M (2015) Cancer-mediated adipose reversion promotes cancer cell migration via IL-6 and MCP-1. Breast Cancer Res Treat 150(2):255–263. https://doi.org/10.1007/s10549-015-3318-2
Li SJ, Wei XH, Zhan XM, He JY, Zeng YQ, Tian XM, Yuan ST, Sun L (2020) Adipocyte-derived leptin promotes PAI-1 -mediated breast cancer metastasis in a STAT3/miR-34a dependent manner. Cancers (Basel) 12(12):3864. https://doi.org/10.3390/cancers12123864
Juárez-Cruz JC, Okoniewski M, Ramírez M, Ortuño-Pineda C, Navarro-Tito N, Castañeda-Saucedo E (2022) Chronic leptin treatment induces epithelial-mesenchymal transition in mcf10a mammary epithelial cells. J Mammary Gland Biol Neoplasia 27(1):19–36. https://doi.org/10.1007/s10911-022-09515-9
Acheva A, Kärki T, Schaible N, Krishnan R, Tojkander S (2021) Adipokine leptin co-operates with mechanosensitive Ca2 +-channels and triggers actomyosin-mediated motility of breast epithelial cells. Front Cell Dev Biol 8:607038. https://doi.org/10.3389/fcell.2020.607038
Olea-Flores M, Zuñiga-Eulogio M, Tacuba-Saavedra A, Bueno-Salgado M, Sánchez-Carvajal A, Vargas-Santiago Y, Mendoza-Catalán MA, Pérez Salazar E, García-Hernández A, Padilla-Benavides T, Navarro-Tito N (2019) Leptin promotes expression of EMT-related transcription factors and invasion in a Src and FAK-dependent pathway in MCF10A mammary epithelial cells. Cells 8(10):1133. https://doi.org/10.3390/cells8101133
Villanueva-Duque A, Zuniga-Eulogio MD, Dena-Beltran J, Castaneda-Saucedo E, Calixto-Galvez M, Mendoza-Catalán MA, Ortuno-Pineda C, Navarro-Tito N (2017) Leptin induces partial epithelial-mesenchymal transition in a FAK-ERK dependent pathway in MCF10A mammary non-tumorigenic cells. Int J Clin Exp Pathol 10(10):10334–10342
Park JW, Zhao L, Willingham MC, Cheng SY (2017) Inhibition of STAT3 signaling blocks obesity-induced mammary hyperplasia in a mouse model. Am J Cancer Res 7(3):727–739
Strong AL, Ohlstein JF, Biagas BA, Rhodes LV, Pei DT, Tucker HA, Llamas C, Bowles AC, Dutreil MF, Zhang S, Gimble JM, Burow ME, Bunnell BA (2015) Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res 17(1):112. https://doi.org/10.1186/s13058-015-0622-z
Haque I, Ghosh A, Acup S, Banerjee S, Dhar K, Ray A, Sarkar S, Kambhampati S, Banerjee SK (2018) Leptin-induced ER-α-positive breast cancer cell viability and migration is mediated by suppressing CCN5-signaling via activating JAK/AKT/STAT-pathway. BMC Cancer 18(1):99. https://doi.org/10.1186/s12885-018-3993-6
Mishra AK, Parish CR, Wong ML, Licinio J, Blackburn AC (2017) Leptin signals via TGFB1 to promote metastatic potential and stemness in breast cancer. PLoS ONE 12(5):e0178454. https://doi.org/10.1371/journal.pone.0178454
Yan D, Avtanski D, Saxena NK, Sharma D (2012) Leptin-induced epithelial-mesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways. J Biol Chem 287(11):8598–8612. https://doi.org/10.1074/jbc.M111.322800
Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD, Rich JN, Hjlemeland AB, Vasanji A, Berger NA, Lathia JD, Reizes O (2013) Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer 20(6):797–808. https://doi.org/10.1530/ERC-13-0329
Duan L, Lu Y, Xie W, Nong L, Jia Y, Tan A, Liu Y (2020) Leptin promotes bone metastasis of breast cancer by activating the SDF-1/CXCR4 axis. Aging (Albany NY) 12(16):16172–16182. https://doi.org/10.18632/aging.103599
Sabol RA, Bowles AC, Côté A, Wise R, O’Donnell B, Matossian MD, Hossain FM, Burks HE, Del Valle L, Miele L, Collins-Burow BM, Burow ME, Bunnell BA (2019) Leptin produced by obesity-altered adipose stem cells promotes metastasis but not tumorigenesis of triple-negative breast cancer in orthotopic xenograft and patient-derived xenograft models. Breast Cancer Res 21(1):67. https://doi.org/10.1186/s13058-019-1153-9
Rios Garcia M, Steinbauer B, Srivastava K, Singhal M, Mattijssen F, Maida A, Christian S, Hess-Stumpp H, Augustin HG, Müller-Decker K, Nawroth PP, Herzig S, Berriel Diaz M (2017) Acetyl-CoA carboxylase 1-dependent protein acetylation controls breast cancer metastasis and recurrence. Cell Metab 26(6):842-855.e5. https://doi.org/10.1016/j.cmet.2017.09.018
Howe EN, Cochrane DR, Richer JK (2011) Targets of miR-200c mediate suppression of cell motility and anoikis resistance. Breast Cancer Res 13(2):R45. https://doi.org/10.1186/bcr2867
Gorrab A, Pagano A, Ayed K, Chebil M, Derouiche A, Kovacic H, Gati A (2021) Leptin promotes prostate cancer proliferation and migration by stimulating STAT3 pathway. Nutr Cancer 73(7):1217–1227. https://doi.org/10.1080/01635581.2020.1792946
Park JW, Han CR, Zhao L, Willingham MC, Cheng SY (2016) Inhibition of STAT3 activity delays obesity-induced thyroid carcinogenesis in a mouse model. Endocr Relat Cancer 23(1):53–63. https://doi.org/10.1530/ERC-15-0417
Trevellin E, Scarpa M, Carraro A, Lunardi F, Kotsafti A, Porzionato A, Saadeh L, Cagol M, Alfieri R, Tedeschi U, Calabrese F, Castoro C, Vettor R (2015) Esophageal adenocarcinoma and obesity: peritumoral adipose tissue plays a role in lymph node invasion. Oncotarget 6(13):11203–11215. https://doi.org/10.18632/oncotarget.3587
Uthaya Kumar DB, Chen CL, Liu JC, Feldman DE, Sher LS, French S, DiNorcia J, French SW, Naini BV, Junrungsee S, Agopian VG, Zarrinpar A, Machida K (2016) TLR4 Signaling via NANOG Cooperates With STAT3 to activate Twist1 and promote formation of tumor-initiating stem-like cells in livers of mice. Gastroenterology 150(3):707–719. https://doi.org/10.1053/j.gastro.2015.11.002
Peng C, Sun Z, Li O, Guo C, Yi W, Tan Z, Jiang B (2019) Leptin stimulates the epithelial-mesenchymal transition and pro-angiogenic capability of cholangiocarcinoma cells through the miR-122/PKM2 axis. Int J Oncol 55(1):298–308. https://doi.org/10.3892/ijo.2019.4807
Harbuzariu A, Gonzalez-Perez RR (2018) Leptin-notch axis impairs 5-fluorouracil effects on pancreatic cancer. Oncotarget 9(26):18239–18253. https://doi.org/10.18632/oncotarget.24435
Feng H, Liu Q, Zhang N, Zheng L, Sang M, Feng J, Zhang J, Wu X, Shan B (2013) Leptin promotes metastasis by inducing an epithelial-mesenchymal transition in A549 lung cancer cells. Oncol Res 21(3):165–171. https://doi.org/10.3727/096504014X13887748696662
Xu M, Cao FL, Li N, Gao X, Su X, Jiang X (2018) Leptin induces epithelial-to-mesenchymal transition via activation of the ERK signaling pathway in lung cancer cells. Oncol Lett 16(4):4782–4788. https://doi.org/10.3892/ol.2018.9230
Gelsomino L, Naimo GD, Catalano S, Mauro L, Andò S (2019) The emerging role of adiponectin in female malignancies. Int J Mol Sci 20(9):2127. https://doi.org/10.3390/ijms20092127
Achari AE, Jain SK (2017) Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci 18(6):1321. https://doi.org/10.3390/ijms18061321
Hwang MS, Yu N, Stinson SY, Yue P, Newman RJ, Allan BB, Dornan D (2013) miR-221/222 targets adiponectin receptor 1 to promote the epithelial-to-mesenchymal transition in breast cancer. PLoS ONE 8(6):e66502. https://doi.org/10.1371/journal.pone.0066502
Li T, Li M, Hu S, Cheng X, Gao Y, Jiang S, Yu Q, Zhang C, Sun P, Xian W, Song Z, Zhang Y, Zheng Q (2017) MiR-221 mediates the epithelial-mesenchymal transition of hepatocellular carcinoma by targeting AdipoR1. Int J Biol Macromol 103:1054–1061. https://doi.org/10.1016/j.ijbiomac.2017.05.108
Tae CH, Kim SE, Jung SA, Joo YH, Shim KN, Jung HK, Kim TH, Cho MS, Kim KH, Kim JS (2014) Involvement of adiponectin in early stage of colorectal carcinogenesis. BMC Cancer 14:811. https://doi.org/10.1186/1471-2407-14-811
Nigro E, Schettino P, Polito R, Scudiero O, Monaco ML, De Palma GD, Daniele A (2018) Adiponectin and colon cancer: evidence for inhibitory effects on viability and migration of human colorectal cell lines. Mol Cell Biochem 448(1–2):125–135. https://doi.org/10.1007/s11010-018-3319-7
Cui E, Guo H, Shen M, Yu H, Gu D, Mao W, Wang X (2018) Adiponectin inhibits migration and invasion by reversing epithelial-mesenchymal transition in non-small cell lung carcinoma. Oncol Rep 40(3):1330–1338. https://doi.org/10.3892/or.2018.6523
Michalakis K, Venihaki M, Mantzoros C, Vazaiou A, Ilias I, Gryparis A, Margioris AN (2015) In prostate cancer, low adiponectin levels are not associated with insulin resistance. Eur J Clin Invest 45(6):572–578. https://doi.org/10.1111/eci.12445
Tan W, Wang L, Ma Q, Qi M, Lu N, Zhang L, Han B (2015) Adiponectin as a potential tumor suppressor inhibiting epithelial-to-mesenchymal transition but frequently silenced in prostate cancer by promoter methylation. Prostate 75(11):1197–1205. https://doi.org/10.1002/pros.23002
Kashiwagi E, Abe T, Kinoshita F, Ushijima M, Masaoka H, Shiota M, Netto GJ, Eto M, Miyamoto H (2020) The role of adipocytokines and their receptors in bladder cancer: expression of adiponectin or leptin is an independent prognosticator. Am J Transl Res 12(6):3033–3045
Sun G, Zhang X, Liu Z, Zhu S, Shen P, Zhang H, Zhang M, Chen N, Zhao J, Chen J, Liu J, Dai J, Wang Z, Zhu X, Wang Y, Zeng H (2019) The Adiponectin-AdipoR1 axis mediates tumor progression and tyrosine kinase inhibitor resistance in metastatic renal cell carcinoma. Neoplasia 21(9):921–931. https://doi.org/10.1016/j.neo.2019.07.004
Deb A, Deshmukh B, Ramteke P, Bhati FK, Bhat MK (2021) Resistin: a journey from metabolism to cancer. Transl Oncol 14(10):101178. https://doi.org/10.1016/j.tranon.2021.101178
Parafiniuk K, Skiba W, Pawłowska A, Suszczyk D, Maciejczyk A, Wertel I (2022) The role of the adipokine resistin in the pathogenesis and progression of epithelial ovarian cancer. Biomedicines 10(4):920. https://doi.org/10.3390/biomedicines10040920
Wang CH, Wang PJ, Hsieh YC, Lo S, Lee YC, Chen YC, Tsai CH, Chiu WC, Chu-Sung HuS, Lu CW, Yang YF, Chiu CC, Ou-Yang F, Wang YM, Hou MF, Yuan SS (2018) Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene 37(5):589–600. https://doi.org/10.1038/onc.2017.357
Kushiro K, Núñez NP (2011) Ob/ob serum promotes a mesenchymal cell phenotype in B16BL6 melanoma cells. Clin Exp Metastasis 28(8):877–886. https://doi.org/10.1007/s10585-011-9418-4
Kuo CH, Chen KF, Chou SH, Huang YF, Wu CY, Cheng DE, Chen YW, Yang CJ, Hung JY, Huang MS (2013) Lung tumor-associated dendritic cell-derived resistin promoted cancer progression by increasing Wolf-Hirschhorn syndrome candidate 1/Twist pathway. Carcinogenesis 34(11):2600–2609. https://doi.org/10.1093/carcin/bgt281
Zhao Y, Zheng R, Ning D, Xie F (2020) MiR-625 inhibits tumor cell invasion, migration and EMT by negatively regulating the expression of resistin in non-small cell lung. Cancer Manag Res 12:4171–4180. https://doi.org/10.2147/CMAR.S248251
Avtanski D, Garcia A, Caraballo B, Thangeswaran P, Marin S, Bianco J, Lavi A, Poretsky L (2019) Resistin induces breast cancer cells epithelial to mesenchymal transition (EMT) and stemness through both adenylyl cyclase-associated protein 1 (CAP1)-dependent and CAP1-independent mechanisms. Cytokine 120:155–164. https://doi.org/10.1016/j.cyto.2019.04.016
Avtanski D, Garcia A, Caraballo B, Thangeswaran P, Marin S, Bianco J, Lavi A, Poretsky L (2019) In vitro effects of resistin on epithelial to mesenchymal transition (EMT) in MCF-7 and MDA-MB-231 breast cancer cells—qRT-PCR and Westen blot analyses data. Data Brief 25:104118
Qiu L, Zhang GF, Yu L, Wang HY, Jia XJ, Wang TJ (2018) Novel oncogenic and chemoresistance-inducing functions of resistin in ovarian cancer cells require miRNAs-mediated induction of epithelial-to-mesenchymal transition. Sci Rep 8(1):12522. https://doi.org/10.1038/s41598-018-30978-6
Audrito V, Messana VG, Deaglio S (2020) NAMPT and NAPRT: two metabolic enzymes with key roles in inflammation. Front Oncol 10:358. https://doi.org/10.3389/fonc.2020.00358
Carbone F, Liberale L, Bonaventura A, Vecchiè A, Casula M, Cea M, Monacelli F, Caffa I, Bruzzone S, Montecucco F, Nencioni A (2017) Regulation and function of extracellular nicotinamide phosphoribosyltransferase/visfatin. Compr Physiol 7(2):603–621. https://doi.org/10.1002/cphy.c160029
Wang YY, Chen HD, Lo S, Chen YK, Huang YC, Hu SC, Hsieh YC, Hung AC, Hou MF, Yuan SF (2020) Visfatin enhances breast cancer progression through CXCL1 induction in tumor-associated macrophages. Cancers (Basel) 12(12):3526. https://doi.org/10.3390/cancers12123526
Soncini D, Caffa I, Zoppoli G, Cea M, Cagnetta A, Passalacqua M, Mastracci L, Boero S, Montecucco F, Sociali G, Lasigliè D, Damonte P, Grozio A, Mannino E, Poggi A, D’Agostino VG, Monacelli F, Provenzani A, Odetti P, Ballestrero A, Bruzzone S, Nencioni A (2014) Nicotinamide phosphoribosyltransferase promotes epithelial-to-mesenchymal transition as a soluble factor independent of its enzymatic activity. J Biol Chem 289(49):34189–34204. https://doi.org/10.1074/jbc.M114.594721
Cao D, Chu L, Xu Z, Gong J, Deng R, Wang B, Zhou S (2021) Visfatin facilitates gastric cancer malignancy by targeting snai1 via the NF-κB signaling. Hum Exp Toxicol 40(10):1646–1655. https://doi.org/10.1177/09603271211006168
Lee J, Kim H, Lee JE, Shin SJ, Oh S, Kwon G, Kim H, Choi YY, White MA, Paik S, Cheong JH, Kim HS (2018) Selective cytotoxicity of the NAMPT inhibitor FK866 toward gastric cancer cells with markers of the epithelial-mesenchymal transition. Due Loss NAPRT Gastroenterol 155(3):799-814.e13. https://doi.org/10.1053/j.gastro.2018.05.024
Yang J, Zhang K, Song H, Wu M, Li J, Yong Z, Jiang S, Kuang X, Zhang T (2016) Visfatin is involved in promotion of colorectal carcinoma malignancy through an inducing EMT mechanism. Oncotarget 7(22):32306–32317. https://doi.org/10.18632/oncotarget.8615
Zhang B, Shi D, Zhang X, Liang G, Liu W, Qiao S (2018) FK866 inhibits the epithelial-mesenchymal transition of hepatocarcinoma MHCC97-H cells. Oncol Lett 16(6):7231–7238. https://doi.org/10.3892/ol.2018.9541
Barraud M, Garnier J, Loncle C, Gayet O, Lequeue C, Vasseur S, Bian B, Duconseil P, Gilabert M, Bigonnet M, Maignan A, Moutardier V, Garcia S, Turrini O, Delpero JR, Giovannini M, Grandval P, Gasmi M, Ouaissi M, Secq V, Poizat F, Guibert N, Iovanna J, Dusetti N (2016) A pancreatic ductal adenocarcinoma subpopulation is sensitive to FK866, an inhibitor of NAMPT. Oncotarget 7(33):53783–53796. https://doi.org/10.18632/oncotarget.10776
Xiao L, Mao Y, Tong Z, Zhao Y, Hong H, Wang F (2021) Radiation exposure triggers the malignancy of non-small cell lung cancer cells through the activation of visfatin/Snail signaling. Oncol Rep 45(3):1153–1161. https://doi.org/10.3892/or.2021.7929
Wang D, Qian G, Wang J, Wang T, Zhang L, Yang P, Lin F (2019) Visfatin is involved in the cisplatin resistance of osteosarcoma cells via upregulation of Snail and Zeb1. Cancer Biol Ther 20(7):999–1006. https://doi.org/10.1080/15384047.2019.1591675
Cheng G, Liu C, Sun X, Zhang L, Liu L, Ouyang J, Li B (2015) Visfatin promotes osteosarcoma cell migration and invasion via induction of epithelial-mesenchymal transition. Oncol Rep 34(2):987–994. https://doi.org/10.3892/or.2015.4053
Audrito V, Messana VG, Moiso E, Vitale N, Arruga F, Brandimarte L, Gaudino F, Pellegrino E, Vaisitti T, Riganti C, Piva R, Deaglio S (2020) NAMPT over-expression recapitulates the braf inhibitor resistant phenotype plasticity in melanoma. Cancers (Basel) 12(12):3855. https://doi.org/10.3390/cancers12123855
Crescenzi E, Leonardi A, Pacifico F (2021) NGAL as a potential target in tumor microenvironment. Int J Mol Sci 22(22):12333. https://doi.org/10.3390/ijms222212333
Jung M, Mertens C, Bauer R, Rehwald C, Brüne B (2017) Lipocalin-2 and iron trafficking in the tumor microenvironment. Pharmacol Res 120:146–156. https://doi.org/10.1016/j.phrs.2017.03.018
Hanai J, Mammoto T, Seth P, Mori K, Karumanchi SA, Barasch J, Sukhatme VP (2005) Lipocalin 2 diminishes invasiveness and metastasis of Ras-transformed cells. J Biol Chem 280(14):13641–13647. https://doi.org/10.1074/jbc.M413047200
Yang J, Bielenberg DR, Rodig SJ, Doiron R, Clifton MC, Kung AL, Strong RK, Zurakowski D, Moses MA (2009) Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci USA 106(10):3913–3918. https://doi.org/10.1073/pnas.0810617106
Kurozumi S, Alsaeed S, Orah N, Miligy IM, Joseph C, Aljohani A, Toss MS, Fujii T, Shirabe K, Green AR, Aleskandarany MA, Rakha EA (2020) Clinicopathological significance of lipocalin 2 nuclear expression in invasive breast cancer. Breast Cancer Res Treat 179(3):557–564. https://doi.org/10.1007/s10549-019-05488-2
Tyagi A, Sharma S, Wu K, Wu SY, Xing F, Liu Y, Zhao D, Deshpande RP, D’Agostino RB Jr, Watabe K (2021) Nicotine promotes breast cancer metastasis by stimulating N2 neutrophils and generating pre-metastatic niche in lung. Nat Commun 12(1):474. https://doi.org/10.1038/s41467-020-20733-9
Lim R, Ahmed N, Borregaard N, Riley C, Wafai R, Thompson EW, Quinn MA, Rice GE (2007) Neutrophil gelatinase-associated lipocalin (NGAL) an early-screening biomarker for ovarian cancer: NGAL is associated with epidermal growth factor-induced epithelio-mesenchymal transition. Int J Cancer 120(11):2426–2434. https://doi.org/10.1002/ijc.22352
Li T, Yu L, Wen J, Liao Q, Liu Z (2016) An early-screening biomarker of endometrial carcinoma: NGAL is associated with epithelio-mesenchymal transition. Oncotarget 7(52):86064–86074. https://doi.org/10.18632/oncotarget.13340
Mannelqvist M, Stefansson IM, Wik E, Kusonmano K, Raeder MB, Øyan AM, Kalland KH, Moses MA, Salvesen HB, Akslen LA (2012) Lipocalin 2 expression is associated with aggressive features of endometrial cancer. BMC Cancer 12:169. https://doi.org/10.1186/1471-2407-12-169
Chung IH, Wu TI, Liao CJ, Hu JY, Lin YH, Tai PJ, Lai CH, Lin KH (2016) Overexpression of lipocalin 2 in human cervical cancer enhances tumor invasion. Oncotarget 7(10):11113–11126. https://doi.org/10.18632/oncotarget.7096
Lu Y, Dong B, Xu F, Xu Y, Pan J, Song J, Zhang J, Huang Y, Xue W (2019) CXCL1-LCN2 paracrine axis promotes progression of prostate cancer via the Src activation and epithelial-mesenchymal transition. Cell Commun Signal 17(1):118. https://doi.org/10.1186/s12964-019-0434-3
Ding G, Fang J, Tong S, Qu L, Jiang H, Ding Q, Liu J (2015) Over-expression of lipocalin 2 promotes cell migration and invasion through activating ERK signaling to increase SLUG expression in prostate cancer. Prostate 75(9):957–968. https://doi.org/10.1002/pros.22978
Falzone L, Candido S, Salemi R, Basile MS, Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M, Libra M (2016) Computational identification of microRNAs associated to both epithelial to mesenchymal transition and NGAL/MMP-9 pathways in bladder cancer. Oncotarget 7(45):72758–72766. https://doi.org/10.18632/oncotarget.11805
Celepli P, Bigat İ, Karabulut S, Celepli S, Hücümenoğlu S (2022) Evaluation of Lipocalin-2 and Twist expression in thyroid cancers and its relationship with epithelial mesenchymal transition. Ann Diagn Pathol 59:151973. https://doi.org/10.1016/j.anndiagpath.2022.151973
Mongre RK, Sodhi SS, Sharma N, Ghosh M, Kim JH, Kim N, Park YH, Shin YG, Kim SJ, Jiao ZJ, Huynh DL (2016) Epigenetic induction of epithelial to mesenchymal transition by LCN2 mediates metastasis and tumorigenesis, which is abrogated by NF-κB inhibitor BRM270 in a xenograft model of lung adenocarcinoma. Int J Oncol 48(1):84–98. https://doi.org/10.3892/ijo.2015.3245
Takegahara K, Usuda J, Inoue T, Sonokawa T, Matsui T, Matsumoto M (2021) Antiaging gene Klotho regulates epithelial-mesenchymal transition and increases sensitivity to pemetrexed by inducing lipocalin-2 expression. Oncol Lett 21(5):418. https://doi.org/10.3892/ol.2021.12679
Guo Y, Zhai J, Zhang J, Zhou H (2020) NGAL protects in nasopharyngeal carcinoma by inducing apoptosis and blocking epithelial-mesenchymal transition. Oncol Lett 19(6):3711–3718. https://doi.org/10.3892/ol.2020.11527
Feng M, Feng J, Chen W, Wang W, Wu X, Zhang J, Xu F, Lai M (2016) Lipocalin2 suppresses metastasis of colorectal cancer by attenuating NF-κB-dependent activation of snail and epithelial mesenchymal transition. Mol Cancer 15(1):77. https://doi.org/10.1186/s12943-016-0564-9
Kim SL, Lee ST, Min IS, Park YR, Lee JH, Kim DG, Kim SW (2017) Lipocalin 2 negatively regulates cell proliferation and epithelial to mesenchymal transition through changing metabolic gene expression in colorectal cancer. Cancer Sci 108(11):2176–2186. https://doi.org/10.1111/cas.13389
Wang YP, Yu GR, Lee MJ, Lee SY, Chu IS, Leem SH, Kim DG (2013) Lipocalin-2 negatively modulates the epithelial-to-mesenchymal transition in hepatocellular carcinoma through the epidermal growth factor (TGF-beta1)/Lcn2/Twist1 pathway. Hepatology 58(4):1349–1361. https://doi.org/10.1002/hep.26467
Chiang KC, Yeh TS, Wu RC, Pang JS, Cheng CT, Wang SY, Juang HH, Yeh CN (2016) Lipocalin 2 (LCN2) is a promising target for cholangiocarcinoma treatment and bile LCN2 level is a potential cholangiocarcinoma diagnostic marker. Sci Rep 6:36138. https://doi.org/10.1038/srep36138
Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, Muruganandan S, Sinal CJ (2007) Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 282(38):28175–28188. https://doi.org/10.1074/jbc.M700793200
Kim H, Lee JH, Lee SK, Song NY, Son SH, Kim KR, Chung WY (2020) Chemerin treatment inhibits the growth and bone invasion of breast cancer cells. Int J Mol Sci 21(8):2871. https://doi.org/10.3390/ijms21082871
Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M (2006) Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443(7112):709–712. https://doi.org/10.1038/nature05162
Stengel A, Mori M, Taché Y (2013) The role of nesfatin-1 in the regulation of food intake and body weight: recent developments and future endeavors. Obes Rev 14(11):859–870. https://doi.org/10.1111/obr.12063
Kan JY, Yen MC, Wang JY, Wu DC, Chiu YJ, Ho YW, Kuo PL (2016) Nesfatin-1/Nucleobindin-2 enhances cell migration, invasion, and epithelial-mesenchymal transition via LKB1/AMPK/TORC1/ZEB1 pathways in colon cancer. Oncotarget 7(21):31336–31349. https://doi.org/10.18632/oncotarget.9140
Tao R, Niu WB, Dou PH, Ni SB, Yu YP, Cai LC, Wang XY, Li SY, Zhang C, Luo ZG (2020) Nucleobindin-2 enhances the epithelial-mesenchymal transition in renal cell carcinoma. Oncol Lett 19(6):3653–3664. https://doi.org/10.3892/ol.2020.11526
Pearsey HM, Henson J, Sargeant JA, Davies MJ, Khunti K, Suzuki T, Bowden-Davies KA, Cuthbertson DJ, Yates TE (2020) Zinc-alpha2-glycoprotein, dysglycaemia and insulin resistance: a systematic review and meta-analysis. Rev Endocr Metab Disord 21(4):569–575. https://doi.org/10.1007/s11154-020-09553-w
Ebadi M, Mazurak VC (2015) Potential biomarkers of fat loss as a feature of cancer cachexia. Mediators Inflamm. https://doi.org/10.1155/2015/820934
Kristensen G, Berg KD, Toft BG, Stroomberg HV, Nolley R, Brooks JD, Brasso K, Roder MA (2019) Predictive value of AZGP1 following radical prostatectomy for prostate cancer: a cohort study and meta-analysis. J Clin Pathol 72(10):696–704. https://doi.org/10.1136/jclinpath-2019-205940
Kong B, Michalski CW, Hong X, Valkovskaya N, Rieder S, Abiatari I, Streit S, Erkan M, Esposito I, Friess H, Kleeff J (2010) AZGP1 is a tumor suppressor in pancreatic cancer inducing mesenchymal-to-epithelial transdifferentiation by inhibiting TGF-β-mediated ERK signaling. Oncogene 29(37):5146–5158. https://doi.org/10.1038/onc.2010.258
Xu MY, Chen R, Yu JX, Liu T, Qu Y, Lu LG (2016) AZGP1 suppresses epithelial-to-mesenchymal transition and hepatic carcinogenesis by blocking TGFβ1-ERK2 pathways. Cancer Lett 374(2):241–249. https://doi.org/10.1016/j.canlet.2016.02.025
Tian H, Ge C, Zhao F, Zhu M, Zhang L, Huo Q, Li H, Chen T, Xie H, Cui Y, Yao M, Li J (2017) Downregulation of AZGP1 by Ikaros and histone deacetylase promotes tumor progression through the PTEN/Akt and CD44s pathways in hepatocellular carcinoma. Carcinogenesis 38(2):207–217. https://doi.org/10.1093/carcin/bgw125
Prentice KJ, Saksi J, Hotamisligil GS (2019) Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J Lipid Res 60(4):734–740. https://doi.org/10.1194/jlr.S091793
Hao J, Zhang Y, Yan X, Yan F, Sun Y, Zeng J, Waigel S, Yin Y, Fraig MM, Egilmez NK, Suttles J, Kong M, Liu S, Cleary MP, Sauter E, Li B (2018) Circulating adipose fatty acid binding protein is a new link underlying obesity-associated breast/mammary tumor development. Cell Metab 28(5):689-705.e5. https://doi.org/10.1016/j.cmet.2018.07.006
Jin J, Zhang Z, Zhang S, Chen X, Chen Z, Hu P, Wang J, Xie C (2018) Fatty acid binding protein 4 promotes epithelial-mesenchymal transition in cervical squamous cell carcinoma through AKT/GSK3β/Snail signaling pathway. Mol Cell Endocrinol 461:155–164. https://doi.org/10.1016/j.mce.2017.09.005
Yu J, Xie Y, Li M, Zhou F, Zhong Z, Liu Y, Wang F, Qi J (2019) Association between SFRP promoter hypermethylation and different types of cancer: a systematic review and meta-analysis. Oncol Lett 18(4):3481–3492. https://doi.org/10.3892/ol.2019.10709
Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K (2010) Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329(5990):454–457. https://doi.org/10.1126/science.1188280
Wang D, Zhang Y, Shen C (2020) Research update on the association between SFRP5, an anti-inflammatory adipokine, with obesity, type 2 diabetes mellitus and coronary heart disease. J Cell Mol Med 24(5):2730–2735. https://doi.org/10.1111/jcmm.15023
Zhou W, Ye C, Li L, Liu L, Wang F, Yu L, Zhou F, Xiang Y, Wang Y, Yin G, Ma Z, Fu Q, Zhang Q, Gao D, Huang S, Yu Z (2020) Adipocyte-derived SFRP5 inhibits breast cancer cells migration and invasion through Wnt and epithelial-mesenchymal transition signaling pathways. Chin J Cancer Res 32(3):347–360. https://doi.org/10.21147/j.issn.1000-9604.2020.03.06
Funding
The authors declare that no funds, grants, or other support were received for this work.
Author information
Authors and Affiliations
Contributions
Conceptualization: HP and IA. IA reviewed the literature, prepared the manuscript and created the figures. HP made the final editing and revision of the manuscript and offered her expert opinion.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akrida, I., Papadaki, H. Adipokines and epithelial-mesenchymal transition (EMT) in cancer. Mol Cell Biochem 478, 2419–2433 (2023). https://doi.org/10.1007/s11010-023-04670-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04670-x