Abstract
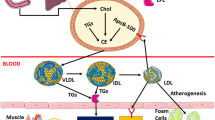
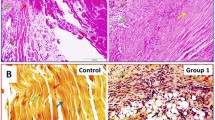
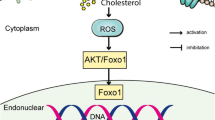
Hyperlipidemia is an important risk factor in the development and progression of tendon pathology, however its role in aggravating rotator cuff tendon injury (RCTI) is largely unknown. We aimed to assess the expression status of key extracellular matrix (ECM) components in the tendon tissues and tenocytes under hyperlipidemia. Shoulder rotator cuff (RC) tendon tissues harvested from the swine model of hyperlipidemia displayed alterations in histomorphometry and the expression status of major ECM component proteins including COL-I, COL-III, COL-IV, COL-V, COL-VI, MMP2, and MMP9. Similarly, the LDL- and oxLDL-challenged tenocytes displayed altered expression of the same proteins at both transcriptional and translational levels. In addition, the lipid uptake and cellular reactive oxygen radicals predominated in the lipid-challenged tenocytes compared to the control. Overall, the LDL-treated cells displayed predominant pathological alterations compared to the ox-LDL-treated cells. Further understanding regarding the underlying molecular mechanisms driving the tendon matrisome alteration and subsequent aggravated RCTI pathology in hyperlipidemia could open novel translational avenues in the management of RCTI.






Similar content being viewed by others
Data availability
Data with the raw counts matrices and annotation are available upon request from the authors through proper channels.
References
Karr S (2017) Epidemiology and management of hyperlipidemia. Am J Manag Care 23:S139–S148
Khosravi M, Hosseini-Fard R, Najafi M (2018) Circulating low density lipoprotein (LDL). Horm Mol Biol Clin Investig. https://doi.org/10.1515/hmbci-2018-0024
Mundi S, Massaro M, Scoditti E, Carluccio MA, Van Hinsbergh VW, Iruela-Arispe ML, De Caterina R (2018) Endothelial permeability, LDL deposition, and cardiovascular risk factors—a review. Cardiovasc Res 114:35–52
Bozkurt B, Aguilar D, Deswal A, Dunbar SB, Francis GS, Horwich T, Jessup M, Kosiborod M, Pritchett AM, Ramasubbu K (2016) Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation 134:e535–e578
Keane WF, Mulcahy WS, Kasiske BL, Kim Y and O'Donnell MP (1991) Hyperlipidemia and progressive renal disease. Kidney International Supplement.
Morton RE, Evans TA (1992) Modification of the bicinchoninic acid protein assay to eliminate lipid interference in determining lipoprotein protein content. Anal Biochem 204:332–334
Karpe F (1997) Mechanisms of postprandial hyperlipidaemia—remnants and coronary artery disease. Diabet Med 14:S60–S66
Ahmad F, Leake DS (2019) Lysosomal oxidation of LDL alters lysosomal pH, induces senescence, and increases secretion of pro-inflammatory cytokines in human macrophages [S]. J Lipid Res 60:98–110
Kovanen PT, Bot I (2017) Mast cells in atherosclerotic cardiovascular disease–Activators and actions. Eur J Pharmacol 816:37–46
Thankam FG, Roesch ZK, Dilisio MF, Radwan MM, Kovilam A, Gross RM, Agrawal DK (2018) Association of inflammatory responses and ECM disorganization with HMGB1 upregulation and NLRP3 inflammasome activation in the injured rotator cuff tendon. Sci Rep 8:1–14
Cancienne JM, Brockmeier SF, Rodeo SA, Werner BC (2017) Perioperative serum lipid status and statin use affect the revision surgery rate after arthroscopic rotator cuff repair. Am J Sports Med 45:2948–2954
Werner B, Cancienne J, Brockmeier S, Rodeo S (2017) Perioperative serum lipid status and statin use affect revision surgery rate after arthroscopic rotator cuff repair. Arthroscopy 33:e26
Lin TT-L, Lin C-H, Chang C-L, Chi C-H, Chang S-T, Sheu WH-H (2015) The effect of diabetes, hyperlipidemia, and statins on the development of rotator cuff disease: a nationwide, 11-year, longitudinal, population-based follow-up study. Am J Sports Med 43:2126–2132
Hast MW, Abboud JA, Soslowsky LJ (2014) Exploring the role of hypercholesterolemia in tendon health and repair. Muscles, Ligaments Tendons J 4:275
Amit P, Kuiper JH, James S, Snow M (2021) Does statin-treated hyperlipidemia affect rotator cuff healing or muscle fatty infiltration after rotator cuff repair? J Shoulder Elbow Surg 30:2465–2474
Yang Y, Qu J (2018) The effects of hyperlipidemia on rotator cuff diseases: a systematic review. J Orthop Surg Res 13:1–11
Steinberg D (2009) The LDL modification hypothesis of atherogenesis: an update. J Lipid Res 50:S376–S381
Dong S, Li J, Zhao H, Zheng Y, Chen Y, Shen J, Yang H, Zhu J (2022) Risk factor analysis for predicting the onset of rotator cuff calcific tendinitis based on artificial intelligence. Computational Intell Neurosci. https://doi.org/10.1155/2022/8978878
Beason DP, Hsu JE, Marshall SM, McDaniel AL, Temel RE, Abboud JA, Soslowsky LJ (2013) Hypercholesterolemia increases supraspinatus tendon stiffness and elastic modulus across multiple species. J Shoulder Elbow Surg 22:681–686
Chung SW, Park H, Kwon J, Choe GY, Kim SH, Oh JH (2016) Effect of hypercholesterolemia on fatty infiltration and quality of tendon-to-bone healing in a rabbit model of a chronic rotator cuff tear: electrophysiological, biomechanical, and histological analyses. Am J Sports Med 44:1153–1164
Thankam FG, Wilson VE, Radwan MM, Siddique A, Agrawal DK (2022) Involvement of ischemia-driven 5-lipoxygenase-resolvin-E1-chemokine like receptor-1 axis in the resolution of post-coronary artery bypass graft inflammation in coronary arteries. Mol Biol Rep 49:3123–3134
Thankam FG, Dilisio MF, Dietz NE, Agrawal DK (2016) TREM-1, HMGB1 and RAGE in the shoulder tendon: dual mechanisms for inflammation based on the coincidence of glenohumeral arthritis. PLoS One 11:e0165492
Stoll C, John T, Conrad C, Lohan A, Hondke S, Ertel W, Kaps C, Endres M, Sittinger M, Ringe J (2011) Healing parameters in a rabbit partial tendon defect following tenocyte/biomaterial implantation. Biomaterials 32:4806–4815
Thankam F, Khwaja B, Nguyen M, Ahsan O and Agrawal D (2022) Acute exposure of minimally ox-LDL elicits survival responses by downregulating the mediators of NLRP3 inflammasome in cultured RAW 264.7 macrophages.
Thankam FG, Ayoub JG, Ahmed MMR, Siddique A, Sanchez TC, Peralta RA, Pennington TJ, Agrawal DK (2021) Association of hypoxia and mitochondrial damage associated molecular patterns in the pathogenesis of vein graft failure: a pilot study. Transl Res 229:38–52
Thankam FG, Chandra IS, Kovilam AN, Diaz CG, Volberding BT, Dilisio MF, Radwan MM, Gross RM, Agrawal DK (2018) Amplification of mitochondrial activity in the healing response following rotator cuff tendon injury. Sci Rep 8:1–14
Tekavec E, Jöud A, Rittner R, Mikoczy Z, Nordander C, Petersson IF, Englund M (2012) Population-based consultation patterns in patients with shoulder pain diagnoses. BMC Musculoskelet Disord 13:1–8
Abboud JA, Kim JS (2010) The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res® 468:1493–1497
Lee C-M, Chien C-T, Chang P-Y, Hsieh M-Y, Jui H-Y, Liau C-S, Hsu S-M, Lee Y-T (2005) High-density lipoprotein antagonizes oxidized low-density lipoprotein by suppressing oxygen free-radical formation and preserving nitric oxide bioactivity. Atherosclerosis 183:251–258
Siess W (2006) Platelet interaction with bioactive lipids formed by mild oxidation of low-density lipoprotein. Pathophysiol Haemost Thromb 35:292–304
Orekhov AN (2018) LDL and foam cell formation as the basis of atherogenesis. Curr Opin Lipidol 29:279–284
Fang WH, Agrawal DK, Thankam FG (2022) “Smart exosomes”: a smart approach for tendon regeneration. Tissue Eng Part B Rev 28:613–625
Rhee S-M, Youn S-M, Ko YW, Kwon TY, Park Y-K, Rhee YG (2021) Retracted rotator cuff repairs heal with disorganized fibrogenesis without affecting biomechanical properties: a comparative animal model study. Arthrosc J Arthrosc Relat Surg. https://doi.org/10.1016/j.arthro.2021.06.025
Gatto AP, Hu DA, Feeley BT, Lansdown D (2022) Dyslipidemia is associated with risk for rotator cuff repair failure: a systematic review and meta-analysis. JSES Rev Rep Tech. https://doi.org/10.1016/j.xrrt.2022.02.003
Savitskaya YA, Izaguirre A, Sierra L, Perez F, Cruz F, Villalobos E, Almazan A, Ibarra C (2011) Effect of angiogenesis-related cytokines on rotator cuff disease: the search for sensitive biomarkers of early tendon degeneration. Clin Med Insights Arthritis Musculoskelet Disord. https://doi.org/10.4137/CMAMD.S7071
Soslowsky LJ, Fryhofer GW (2016) Tendon homeostasis in hypercholesterolemia. Metab Influences Risk Tendon Disord. https://doi.org/10.1007/978-3-319-33943-6_14
Izumi S, Oichi T, Shetye SS, Zhang K, Wilson K, Iwamoto M, Kuo CK, Akabudike N, Adachi N, Soslowsky LJ (2022) Inhibition of glucose use improves structural recovery of injured Achilles tendon in mice. J Orthop Res® 40:1409–1419
Chaudhury S, Xia Z, Thakkar D, Hakimi O, Carr AJ (2016) Gene expression profiles of changes underlying different-sized human rotator cuff tendon tears. J Shoulder Elbow Surg 25:1561–1570
Thankam FG, Evan DK, Agrawal DK, Dilisio MF (2019) Collagen type III content of the long head of the biceps tendon as an indicator of glenohumeral arthritis. Mol Cell Biochem 454:25–31
Zhang G, Young B, Ezura Y, Favata M, Soslowsky L, Chakravarti S, Birk DE (2005) Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact 5:5–21
Thankam FG, Dilisio MF, Gross RM, Agrawal DK (2018) Collagen I: a kingpin for rotator cuff tendon pathology. Am J Translational Res 10:3291
Kjær M, Langberg H, Heinemeier K, Bayer M, Hansen M, Holm L, Doessing S, Kongsgaard M, Krogsgaard M, Magnusson S (2009) From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports 19:500–510
Eriksen HA, Pajala A, Leppilahti J, Risteli J (2002) Increased content of type III collagen at the rupture site of human Achilles tendon. J Orthop Res 20:1352–1357
Gonçalves-Neto J, Witzel S, Teodoro W, Carvalho-Junior A, Fernandes TD, Yoshinari H (2002) Changes in collagen matrix composition in human posterior tibial tendon dysfunction. Joint Bone Spine 69:189–194
Izu Y, Ansorge HL, Zhang G, Soslowsky LJ, Bonaldo P, Chu M-L, Birk DE (2011) Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice. Matrix Biol 30:53–61
Smith SM, Thomas CE, Birk DE (2012) Pericellular proteins of the developing mouse tendon: a proteomic analysis. Connect Tissue Res 53:2–13
Ritty TM, Roth R, Heuser JE (2003) Tendon cell array isolation reveals a previously unknown fibrillin-2-containing macromolecular assembly. Structure 11:1179–1188
Sardone F, Santi S, Tagliavini F, Traina F, Merlini L, Squarzoni S, Cescon M, Wagener R, Maraldi NM, Bonaldo P (2016) Collagen VI–NG2 axis in human tendon fibroblasts under conditions mimicking injury response. Matrix Biol 55:90–105
Theocharidis G, Drymoussi Z, Kao AP, Barber AH, Lee DA, Braun KM, Connelly JT (2016) Type VI collagen regulates dermal matrix assembly and fibroblast motility. J Investig Dermatol 136:74–83
Subramanian A, Schilling TF (2015) Tendon development and musculoskeletal assembly: emerging roles for the extracellular matrix. Development 142:4191–4204
Aimes RT, Quigley JP (1995) Matrix metalloproteinase-2 is an interstitial collagenase: inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type i collagen generating the specific ¾-and ¼-length fragments (∗). J Biol Chem 270:5872–5876
Koskinen SO, Heinemeier KM, Olesen JL, Langberg H, Kjaer M (2004) Physical exercise can influence local levels of matrix metalloproteinases and their inhibitors in tendon-related connective tissue. J Appl Physiol 96:861–864
Andarawis-Puri N, Sereysky JB, Sun HB, Jepsen KJ, Flatow EL (2012) Molecular response of the patellar tendon to fatigue loading explained in the context of the initial induced damage and number of fatigue loading cycles. J Orthop Res 30:1327–1334
Attia M, Huet E, Gossard C, Menashi S, Tassoni M-C, Martelly I (2013) Early events of overused supraspinatus tendons involve matrix metalloproteinases and EMMPRIN/CD147 in the absence of inflammation. Am J Sports Med 41:908–917
Huisman E, Lu A, Jamil S, Mousavizadeh R, McCormack R, Roberts C, Scott A (2016) Influence of repetitive mechanical loading on MMP2 activity in tendon fibroblasts. J Orthop Res 34:1991–2000
Loiselle AE, Bragdon GA, Jacobson JA, Hasslund S, Cortes ZE, Schwarz EM, Mitten DJ, Awad HA, O’Keefe RJ (2009) Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. J Orthop Res 27:833–840
Karousou E, Ronga M, Vigetti D, Passi A, Maffulli N (2008) Collagens, proteoglycans, MMP-2, MMP-9 and TIMPs in human achilles tendon rupture. Clin Orthop Relat Res 466:1577–1582
Eliasberg CD, Wada S, Carballo CB, Nakagawa Y, Nemirov DA, Bhandari R, Otero M, Deng XH, Rodeo SA (2019) Identification of inflammatory mediators in tendinopathy using a murine subacromial impingement model. J Orthop Res® 37:2575–2582
Lakemeier S, Braun J, Efe T, Foelsch C, Archontidou-Aprin E, Fuchs-Winkelmann S, Paletta JR, Schofer MD (2011) Expression of matrix metalloproteinases 1, 3, and 9 in differing extents of tendon retraction in the torn rotator cuff. Knee Surg Sports Traumatol Arthrosc 19:1760–1765
Grewal N, Thornton GM, Behzad H, Sharma A, Lu A, Zhang P, Reid WD, Granville DJ, Scott A (2014) Accumulation of oxidized LDL in the tendon tissues of C57BL/6 or apolipoprotein E knock-out mice that consume a high fat diet: potential impact on tendon health. PLoS ONE 9:e114214
Beason DP, Abboud JA, Kuntz AF, Bassora R, Soslowsky LJ (2011) Cumulative effects of hypercholesterolemia on tendon biomechanics in a mouse model. J Orthop Res 29:380–383
Bestwick C, Maffulli N (2004) Reactive oxygen species and tendinopathy: do they matter? Br J Sports Med 38:672–674
Thankam FG, Diaz C, Chandra I, Link J, Newton J, Dilisio MF, Agrawal DK (2022) Hybrid interpenetrating hydrogel network favoring the bidirectional migration of tenocytes for rotator cuff tendon regeneration. J Biomed Mater Res B Appl Biomater 110:467–477
Acknowledgements
FGT is thankful to WU for the startup funds and DKA is grateful to National Institutes of Health, USA for funding.
Funding
The research work of FGT is supported by the startup funds from WU and DK Agrawal is supported by research grants R01 HL144125 and R01HL147662 from the National Institutes of Health, USA.
Author information
Authors and Affiliations
Contributions
WF: experimental design; data generation; data analysis; manuscript preparation; preparation of figures; manuscript editing. SS: data generation; data analysis; manuscript editing. DT: data generation; data analysis; manuscript editing. CF: data generation; data analysis; manuscript editing. VL: data generation; data analysis; manuscript editing. CD: data generation; manuscript editing;. CD: data generation; manuscript editing. DKA: Conceptualization; experimental design, manuscript preparation; manuscript editing; resources; funding. FGT: Conceptualization; experimental design, data generation; data analysis; manuscript preparation; manuscript editing; resources; funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
competing interest
All the authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Consent for publication
All the authors have read the manuscript and consented for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, W., Sekhon, S., Teramoto, D. et al. Pathological alterations in the expression status of rotator cuff tendon matrix components in hyperlipidemia. Mol Cell Biochem 478, 1887–1898 (2023). https://doi.org/10.1007/s11010-022-04643-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04643-6