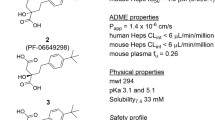
Abstract
The sodium dependent SLC13 family transporters comprise of five genes SLC13A1, SLC13A2 (NaDC1), SLC13A3 (NaDC3), SLC13A4 and SLC13A5 (NaCT). Among them, NaDC1, NaDC3 and NaCT are sodium dependent transporters belonging to family of dicarboxylates (succinate, malate, α-ketoglutarate) and tricarboxylates (citrate). The mouse and the human NaCT structures have still not been crystallized, therefore structural information is taken from the related bacterial transporter of VcINDY. Citrate in the cytosol works as a precursor for the fatty acid synthesis, cholesterol, and low-density lipoproteins. The excess citrate from the matrix is translocated to the cytosol for fatty acid synthesis through these transporters and thus controls the energy balance by downregulating the glycolysis, tricarboxylic acid (TCA), and fatty acid breakdown. These transporters play an important role in regulating various metabolic diseases including cancer, diabetes, obesity, fatty liver diseases and CNS disorders. These di and tricarboxylate transporters are emerging as new targets for metabolic disorders such as obesity and diabetes. The mutation in the function of the NaCT causes several neurological diseases including neonatal epilepsy and impaired brain development whereas mutation of genes coding for citrate transport present in the liver may provide positive effect. Therefore, continued efforts from the earlier work on citrate transporters are required for the development of citrate inhibitors. This review discusses the structure, function, and regulation of the NaCT transporter. The review also highlights citrate role in diagnosing diseases such as cancer, diabetes, fatty liver, and diabetes. The therapeutic perspective of synthetic inhibitors against NaCT transporters is succinctly summarized.
Graphical abstract

















Similar content being viewed by others
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
Colas C, Pajor AM, Schlessinger A (2015) Structure-Based identification of inhibitors for the SLC13 Family of Na+/Dicarboxylate cotransporters. Biochemistry 54(31):4900–4908
Srisawang P, Chatsudthipong A, Chatsudthipong V (2007) Modulation of succinate transport in Hep G2 cell line by PKC. Biochim Biophys Acta (BBA) 1768(6):1378–1388
Mycielska ME, Patel A, Rizaner N, Mazurek MP, Keun H, Patel A et al (2009) Citrate transport and metabolism in mammalian cells: prostate epithelial cells and prostate cancer. BioEssays 31(1):10–20
Jaramillo-Martinez V, Urbatsch IL, Ganapathy V (2020) Functional distinction between human and mouse sodium-coupled citrate transporters and its biologic significance: an attempt for structural basis using a homology modeling approach. Chem Rev 121(9):5359–5377
Yang J, Li S, Kabir Khan MA, Garre V, Vongsangnak W, Song Y (2019) Increased lipid accumulation in Mucor circinelloides by overexpression of mitochondrial citrate transporter genes. Ind Eng Chem Res 58(6):2125–2134
Yang J, Cánovas-Márquez JT, Li P, Li S, Niu J, Wang X et al (2021) Deletion of plasma membrane malate transporters increased lipid accumulation in the oleaginous fungus mucor circinelloides WJ11. J Agric Food Chem 69(33):9632–9641
Pos KM, Dimroth P (1996) Functional properties of the purified Na+-dependent citrate carrier of Klebsiella pneumoniae: evidence for asymmetric orientation of the carrier protein in proteoliposomes. Biochem 35(3):1018–1026
Sauer DB, Song J, Wang B, Hilton JK, Karpowich NK, Mindell JA et al (2021) Structure and inhibition mechanism of the human citrate transporter NaCT. Nature 591(7848):157–161
Starkov AA, Chinopoulos C, Starkova NN, Konrad C, Kiss G, Stepanova A et al (2017) Divalent cation chelators citrate and EDTA unmask an intrinsic uncoupling pathway in isolated mitochondria. J Bioenerg Biomembr 49(1):3–11
Pajor AM (2006) Molecular properties of the SLC13 family of dicarboxylate and sulfate transporters. Pflugers Arch 451(5):597–605
Bergeron M, Clemençon B, Hediger M, Markovich D (2013) SLC13 family of Na+-coupled di-and tri-carboxylate/sulfate transporters. Mol Asp Med 34(2–3):299–312
Jaramillo-Martinez V, Ganapathy V, Urbatsch IL (2021) A home run for human NaCT/SLC13A5/INDY: cryo-EM structure and homology model to predict transport mechanisms, inhibitor interactions and mutational defects. Biochem J 478(11):2051–2057
Mancusso R, Gregorio GG, Liu Q, Wang D-N (2012) Structure and mechanism of a bacterial sodium-dependent dicarboxylate transporter. Nature 491(7425):622–626
Ganapathy VM, GA 30907 (US), Inoue, Katsuhisa; Nagoya 467–0056 (JP), Fei, You-Jun North Augusta, SC 29841 (US) (2007) Nact as a target for lifespan expansion and weight reduction. European Patent Application 1 816 139 A1.
Khamaysi A, Aharon S, Eini-Rider H, Ohana E (2020) A dynamic anchor domain in slc13 transporters controls metabolite transport. J Biol Chem 295(24):8155–8163
Mulligan C, Fenollar-Ferrer C, Fitzgerald GA, Vergara-Jaque A, Kaufmann D, Li Y et al (2016) The bacterial dicarboxylate transporter VcINDY uses a two-domain elevator-type mechanism. Nat Struct Mol Biol 23(3):256–263
Sauer DB, Wang B, Sudar JC, Song J, Marden J, Rice WJ et al (2022) The ups and downs of elevator-type di-/tricarboxylate membrane transporters. FEBS J 289(6):1515–1523
Pajor AM (2014) Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflugers Arch 466(1):119–130
Huard K, Gosset JR, Montgomery JI, Gilbert A, Hayward MM, Magee TV et al (2016) Optimization of a dicarboxylic series for in vivo inhibition of citrate transport by the solute carrier 13 (SLC13) family. J Med Chem 59(3):1165–1175
Inoue K, Zhuang L, Maddox DM, Smith SB, Ganapathy V (2002) Structure, function, and expression pattern of a novel sodium-coupled citrate transporter (NaCT) cloned from mammalian brain. J Bio Chem 277(42):39469–39476
Inoue K, Zhuang L, Ganapathy V (2002) Human Na+-coupled citrate transporter: primary structure, genomic organization, and transport function. Biophys Res Commun 299(3):465–471
Wada M, Shimada A, Fujita T (2006) Functional characterization of Na+-coupled citrate transporter NaC2/NaCT expressed in primary cultures of neurons from mouse cerebral cortex. Brain Res 1081(1):92–100
Klotz J, Porter BE, Colas C, Schlessinger A, Pajor AM (2016) Mutations in the Na+/citrate cotransporter NaCT (SLC13A5) in pediatric patients with epilepsy and developmental delay. Mol Med 22(1):310–321
Selch S, Chafai A, Sticht H, Birkenfeld AL, Fromm MF, König J (2018) Analysis of naturally occurring mutations in the human uptake transporter NaCT important for bone and brain development and energy metabolism. Sci Rep 8(1):1–12
Bandell M, Lolkema JS (1999) Stereoselectivity of the membrane potential-generating citrate and malate transporters of lactic acid bacteria. Biochem 38(32):10352–10360
Bandell M, Lolkema JS (2000) The conserved C-terminus of the citrate (CitP) and malate (MleP) transporters of lactic acid bacteria is involved in substrate recognition. Biochem 39(42):13059–13067
Sobczak I, Lolkema JS (2005) Loop VIII/IX of the Na+-citrate transporter CitS of Klebsiella pneumoniae folds into an amphipathic surface helix. Biochem 44(14):5461–5470
Higuchi K, Kopel JJ, Sivaprakasam S, Jaramillo-Martinez V, Sutton RB, Urbatsch IL et al (2020) Functional analysis of a species-specific inhibitor selective for human Na+-coupled citrate transporter (NaCT/SLC13A5/mINDY). Biochem J 477(21):4149–4165
Pajor AM, de Oliveira CA, Song K, Huard K, Shanmugasundaram V, Erion DM (2016) Molecular basis for inhibition of the Na+/citrate transporter NaCT (SLC13A5) by dicarboxylate inhibitors. Mol Pharmacol 90(6):755–765
Kopel J, Higuchi K, Ristic B, Sato T, Ramachandran S, Ganapathy V (2020) The hepatic plasma membrane citrate transporter NaCT (SLC13A5) as a molecular target for metformin. Sci Rep 10(1):1–12
Pajor AM, Sun NN (2013) Nonsteroidal anti-inflammatory drugs and other anthranilic acids inhibit the Na+/dicarboxylate symporter from Staphylococcus aureus. Biochem 52(17):2924–2932
Aluvila S, Sun J, Harrison DH, Walters DE, Kaplan RS (2010) Inhibitors of the mitochondrial citrate transport protein: validation of the role of substrate binding residues and discovery of the first purely competitive inhibitor. Mol Pharmacol 77(1):26–34
Colas C, Schlessinger A, Pajor AM (2017) Mapping functionally important residues in the Na+/dicarboxylate cotransporter, NaDC1. Biochem 56(33):4432–4441
Pajor AM, Sun NN (2010) Role of isoleucine-554 in lithium binding by the Na+/dicarboxylate cotransporter NaDC1. Biochem 49(41):8937–8943
Burckhardt BC, Lorenz J, Kobbe C, Burckhardt G (2005) Substrate specificity of the human renal sodium dicarboxylate cotransporter, hNaDC-3, under voltage-clamp conditions. Am J Physiol Renal Physiol 288(4):F792–F799
Bhutia YD, Kopel JJ, Lawrence JJ, Neugebauer V, Ganapathy V (2017) Plasma membrane Na+-coupled citrate transporter (SLC13A5) and neonatal epileptic encephalopathy. Molecules 22(3):378
Nota B, Struys EA, Pop A, Jansen EE, Ojeda MRF, Kanhai WA et al (2013) Deficiency in SLC25A1, encoding the mitochondrial citrate carrier, causes combined D-2-and L-2-hydroxyglutaric aciduria. Am J Hum Genet 92(4):627–631
Mosaoa R, Kasprzyk-Pawelec A, Fernandez HR, Avantaggiati ML (2021) The mitochondrial citrate carrier SLC25A1/CIC and the fundamental role of citrate in cancer, inflammation and beyond. Biomolecules 11(2):141
Mosaoa RM (2021) Role of the mitochondrial citrate transporter, SLC25A1, in cancer obesity and liver steatosis. Georgetown University, Washington
Tan M, Mosaoa R, Graham GT, Kasprzyk-Pawelec A, Gadre S, Parasido E et al (2020) Inhibition of the mitochondrial citrate carrier, Slc25a1, reverts steatosis, glucose intolerance, and inflammation in preclinical models of NAFLD/NASH. Cell Death Differ 27(7):2143–2157
Patil SA, Mayor JA, Kaplan RS (2022) Citrate transporter inhibitors: possible new anticancer agents. Future Med Chem 14(9):665–679
Geissler E, Maria M, Ruemmele P (2016) Plasma membrane citrate transporter for use in the diagnosis and treatment of cancer. Eur Pat App 17(003):750
Parkinson EK, Adamski J, Zahn G, Gaumann A, Flores-Borja F, Ziegler C et al (2021) Extracellular citrate and metabolic adaptations of cancer cells. Cancer Metastasis Rev 40(4):1073–1091
Devi Khwairakpam A, Singh Shyamananda M, Lalduhsaki Sailo B, Raju Rathnakaram S, Padmavathi G, Kotoky J et al (2015) ATP citrate lyase (ACLY): a promising target for cancer prevention and treatment. Curr Drug Targets 16(2):156–163
Philippe I, Hubert L (2016) The reduced concentration of citrate in cancer cells: an indicator of cancer aggressiveness and a possible therapeutic target. Drug Resist Updat 29:47–53
Kumari R, Deshmukh RS, Das S (2019) Caspase-10 inhibits ATP-citrate lyase-mediated metabolic and epigenetic reprogramming to suppress tumorigenesis. Nat Commun 10(1):1–15
Drexler K, Schmidt KM, Jordan K, Federlin M, Milenkovic VM, Liebisch G et al (2021) Cancer-associated cells release citrate to support tumour metastatic progression. Life Sci Alliance 4(6):1–19
Icard P, Coquerel A, Wu Z, Gligorov J, Fuks D, Fournel L et al (2021) Understanding the central role of citrate in the metabolism of cancer cells and tumors: an update. I Int J Mol Sci 22(12):6587
Ren J-G, Seth P, Ye H, Guo K, Hanai J-i, Husain Z et al (2017) Citrate suppresses tumor growth in multiple models through inhibition of glycolysis, the tricarboxylic acid cycle and the IGF-1R pathway. Sci Rep 7(1):1–13
Icard P, Simula L, Wu Z, Berzan D, Sogni P, Dohan A et al (2021) Why may citrate sodium significantly increase the effectiveness of transarterial chemoembolization in hepatocellular carcinoma? Drug Resist Updat. https://doi.org/10.1016/j.drup.2021.100790
Infantino V, Pierri CL, Iacobazzi V (2019) Metabolic routes in inflammation: the citrate pathway and its potential as therapeutic target. Curr Med Chem 26(40):7104–7116
Infantino V, Convertini P, Cucci L, Panaro MA, Di Noia MA, Calvello R et al (2011) The mitochondrial citrate carrier: a new player in inflammation. Biochem J 438(3):433–436
Ashbrook M, McDonough K, Pituch J, Christopherson P, Cornell T, Selewski D et al (2015) Citrate modulates lipopolysaccharide-induced monocyte inflammatory responses. Clin Exp Immunol 180(3):520–530
Choi E-Y, Kim H-J, Han J-S (2015) Anti-inflammatory effects of calcium citrate in RAW 264.7 cells via suppression of NF-κB activation. Environ Toxicol Pharmacol 39(1):27–34
Santarsiero A, Leccese P, Convertini P, Padula A, Abriola P, D’Angelo S et al (2018) New insights into Behçet’s syndrome metabolic reprogramming: citrate pathway dysregulation. Mediators Inflamm. https://doi.org/10.1155/2018/1419352
Williams NC, O’Neill LA (2018) A role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front Immunol 9:141
Henke C, Töllner K, van Dijk RM, Miljanovic N, Cordes T, Twele F et al (2020) Disruption of the sodium-dependent citrate transporter SLC13A5 in mice causes alterations in brain citrate levels and neuronal network excitability in the hippocampus. Neurobiol Dis 143:105018
Rigby MJ, Orefice NS, Lawton AJ, Ma M, Shapiro SL, Yi SY et al (2022) SLC13A5/sodium-citrate co-transporter overexpression causes disrupted white matter integrity and an autistic-like phenotype. Brain comm 4(1):fcac002
Westergaard N, Waagepetersen HS, Belhage B, Schousboe A (2017) Citrate, a ubiquitous key metabolite with regulatory function in the CNS. Neurochem Res 42(6):1583–1588
Kumar A, Cordes T, Thalacker-Mercer AE, Pajor AM, Murphy AN, Metallo CM (2021) NaCT/SLC13A5 facilitates citrate import and metabolism under nutrient-limited conditions. Cell Rep 36(11):109701
Napoli E, Tassone F, Wong S, Angkustsiri K, Simon TJ, Song G et al (2015) Mitochondrial citrate transporter-dependent metabolic signature in the 22q11. 2 deletion syndrome. J Bio Chem 290(38):23240–23253
Siculella L, Giannotti L, Testini M, Gnoni GV, Damiano F (2020) In steatotic cells, ATP-citrate lyase mRNA is efficiently translated through a cap-independent mechanism, contributing to the stimulation of de novo lipogenesis. Int J Mol Sci 21(4):1206
Guo L, Guo Y-Y, Li B-Y, Peng W-Q, Chang X-X, Gao X et al (2019) Enhanced acetylation of ATP-citrate lyase promotes the progression of nonalcoholic fatty liver disease. J Bio Chem 294(31):11805–11816
Wang Q, Jiang L, Wang J, Li S, Yu Y, You J et al (2009) Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology 49(4):1166–1175
van de Wier B, Balk JM, Haenen GR, Giamouridis D, Bakker JA, Bast BC et al (2013) Elevated citrate levels in non-alcoholic fatty liver disease: the potential of citrate to promote radical production. FEBS lett 587(15):2461–2466
Sun Q, Niu Q, Guo Y, Zhuang Y, Li X, Liu J et al (2021) Regulation on citrate influx and metabolism through inhibiting SLC13A5 and ACLY: a novel mechanism mediating the therapeutic effects of curcumin on NAFLD. J Agri Food Chem 69(31):8714–8725
Arefhosseini S, Tutunchi H, Golzar S, Mahboob S, Pouretedal Z, Ebrahimi-Mameghani M (2022) The effect of hydroxy citric acid supplementation with calorie-restricted diet on metabolic, atherogenic and inflammatory biomarkers in women with non-alcoholic fatty liver disease: a randomized controlled clin trial. Food Funct 13(9):5124–5134
Chen Y, Deb DK, Fu X, Yi B, Liang Y, Du J et al (2019) ATP-citrate lyase is an epigenetic regulator to promote obesity-related kidney injury. The FASEB J 33(8):9602–9615
Tomar M, Rao RP, Dorairaj P, Koshta A, Suresh S, Rafiq M et al (2019) A clinical and computational study on anti-obesity effects of hydroxycitric acid. RSC Adv 9(32):18578–18588
Christe M, Hirzel E, Lindinger A, Kern B, von Flüe M, Peterli R et al (2013) Obesity affects mitochondrial citrate synthase in human omental adipose tissue. Int Sch Res Notices. https://doi.org/10.1155/2013/826027
Spencer AF, Lowenstein J (1966) Citrate and the conversion of carbohydrate into fat. citrate cleavage in obesity and lactation. Biochem J 99(3):760
Leandro JG, Espindola-Netto JM, Vianna MCF, Gomez LS, DeMaria TM, Marinho-Carvalho MM et al (2016) Exogenous citrate impairs glucose tolerance and promotes visceral adipose tissue inflammation in mice. Bri J Nut 115(6):967–973
Sui* W, Calvert JK, Kavoussi NL, Asplin J, Miller NL, Bejan CA, et al. (2020) MP03-05 Urinary citrate wasting among nephrolithiasis patients associates with obesity and diabetes mellitus. J Urol 203(Supplement 4):e23-e.
Zahn GB, Steve, Yarnold, Chris, Schaertl, Sabine; Khor, Someina (2018) Inhibitors of citrate transporter and their use in therapy. World Intellectual Property Organization; Patent Cooperation Treaty (PCT) WO 2018/172251 A1.
Alhindi Y, Vaanholt LM, Al-Tarrah M, Gray SR, Speakman JR, Hambly C et al (2019) Low citrate synthase activity is associated with glucose intolerance and lipotoxicity. J Nut Met. https://doi.org/10.1155/2019/8594825
Mohamed E, Hanan NG, Imam RAN, Basma EMS (2020) Possible protective role of citrate against apoptosis and disruption of intercalated disc integrity in a rat model of diabetic cardiomyopathy. The Med J Cairo Univ 88:589–597
Gnoni GV, Giudetti AM, Mercuri E, Damiano F, Stanca E, Priore P et al (2010) Reduced activity and expression of mitochondrial citrate carrier in streptozotocin-induced diabetic rats. Endocrinology 151(4):1551–1559
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
MJA: supervision, writing—original draft; SAK: resources, writing—review & editing; BK: writing—review & editing; PC: writing—review & editing; RB: writing—review & editing; KS: writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The review article requires no ethical approval.
Consent to participation
NA.
Consent to publication
NA.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akhtar, M.J., Khan, S.A., Kumar, B. et al. Role of sodium dependent SLC13 transporter inhibitors in various metabolic disorders. Mol Cell Biochem 478, 1669–1687 (2023). https://doi.org/10.1007/s11010-022-04618-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04618-7