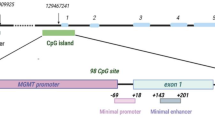
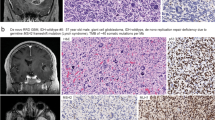
Abstract
We aimed to understand the crosstalk between mismatch repair (MMR) and FA-BRCA pathway in primary bladder carcinoma (BlCa) samples as well as in chemotolerant cell line. We analysed the genetic alterations of MLH1 and MSH2 (MMR-related genes) and after that we correlated it with the nuclear translocation of FANCD2 protein. Next, we evaluated this crosstalk in T24 BlCa cell line in response to doxorubicin treatment. In primary BlCa tumors, infrequent genetic deletion (17–20%) but frequent promoter methylation (28–55%) of MLH1 and MSH2 was observed, where MLH1 was significantly (p < 0.05) more methylated among the early staged samples (NMIBC). However, MSH2 was significantly more altered among the NMIBC samples, signifying the importance of MMR pathway during the early pathogenesis of the disease. Furthermore, BlCa samples with underexpressed MLH1/MSH2 protein possessed cytoplasmic FANCD2 protein; encouraging that inefficiency of MMR proteins might restrict FANCD2 nuclear translocation. Next, we analysed publicly available data in GEO2R tool where we observed that in response to chemotherapeutic drugs, expression of MLH1, MSH2 and FANCD2 were diminishing. Validating this result in doxorubicin tolerant T24 cells, we found that expression of MLH1 and MSH2 was gradually decreased with increasing dose of doxorubicin. Interestingly, FANCD2 mono-ubiquitination (L-form) was also reduced in chemotolerant T24 cells. The crosstalk between MMR and FA-BRCA pathway was substantiated in the primary BlCa tumors. Further, in response to doxorubicin, this crosstalk was found to be hampered due to under-expression of MLH1 and MSH2 gene, thereby rendering chemotolerance.






Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
Change history
02 May 2024
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s11010-024-05021-0
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clinicians 68(6):394–424
Schabath MB, Spitz MR, Grossman HB et al (2003) Genetic instability in bladder cancer assessed by the comet assay. J Natl Cancer Inst 95(7):540–547
Yamamoto Y, Matsuyama H, Chochi Y et al (2007) Overexpression of BUBR1 is associated with chromosomal instability in bladder cancer. Cancer Genet Cytogenet 174(1):42–47
Knowles MA, Hurst CD (2015) Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 15(1):25–41
Sanguedolce F, Cormio A, Massenio P et al (2018) Altered expression of HER-2 and the mismatch repair genes MLH1 and MSH2 predicts the outcome of T1 high-grade bladder cancer. J Cancer Res Clin Oncol 144(4):637–644
Eichenauer T, Bannenberg DC, Wittmer MC et al (2020) 8p deletions in renal cell carcinoma are associated with unfavorable tumor features and poor overall survival. Urol Oncol 38(2):43
Panneerselvam J, Park HW, Zhang J et al (2012) FAVL impairment of the fanconi anemia pathway promotes the development of human bladder cancer. Cell Cycle 11(15):2947–2955
Neveling K, Kalb R, Florl AR et al (2007) Disruption of the FA/BRCA pathway in bladder cancer. Cytogenet Genome Res 118(2–4):166–176
Zhang F, Fan Q, Ren K et al (2010) FANCJ/BRIP1 recruitment and regulation of FANCD2 in DNA damage responses. Chromosoma 119(6):637–649
Basu M, Ghosh S, Roychowdhury A et al (2020) Integrative genomics and pathway analysis identified prevalent FA-BRCA pathway alterations in arsenic-associated urinary bladder carcinoma: chronic arsenic accumulation in cancer tissues hampers the FA-BRCA pathway. Genomics 112(6):5055–5065
Li J, Liu H, Yu J et al (2015) Chemoresistance to doxorubicin induces epithelial-mesenchymal transition via upregulation of transforming growth factor β signaling in HCT116 colon cancer cells. Mol Med Rep 12(1):192–198
Wang Y, Wiltshire T, Senft J et al (2006) Fanconi anemia D2 protein confers chemoresistance in response to the anticancer agent, irofulven. Mol Cancer Ther 5(12):3153–3161
Nakashima S, Kobayashi S, Nagano H et al (2015) BRCA/fanconi anemia pathway implicates chemoresistance to gemcitabine in biliary tract cancer. Cancer Sci 106(5):584–591
Hashimoto T, Kurokawa Y, Takahashi T et al (2019) Predictive value of MLH1 and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Gastric Cancer 22(4):785–792
Basu M, Chatterjee A, Chakraborty B et al (2021) High nuclear expression of HIF1α, synergizing with inactivation of LIMD1 and VHL, portray worst prognosis among the bladder cancer patients: association with arsenic prevalence. J Cancer Res Clin Oncol 147(8):2309–2322
Dasgupta H, Islam S, Alam N et al (2019) Hypomethylation of mismatch repair genes MLH1 and MSH2 is associated with chemotolerance of breast carcinoma: clinical significance. J Surg Oncol 119(1):88–100
Roy R, Mandal S, Chakrabarti J et al (2021) Downregulation of hyaluronic acid-CD44 signaling pathway in cervical cancer cell by natural polyphenols plumbagin pongapin and karanjin. Mol Cell Biochem 476(10):3701–3709
Islam S, Dasgupta H, Basu M et al (2020) Downregulation of beta-catenin in chemo-tolerant TNBC through changes in receptor and antagonist profiles of the WNT pathway: clinical and prognostic implications. Cell Oncol 43(4):725–741
Ling YH, el-Naggar AK, Priebe W et al (1996) Cell cycle-dependent cytotoxicity, G2/M phase arrest, and disruption of p34cdc2/cyclin B1 activity induced by doxorubicin in synchronized P388 cells. Mol Pharmacol 49(5):832–841
Hayashi T, Fujita K, Hayashi Y et al (2020) Mutational landscape and environmental effects in bladder cancer. Int J Mol Sci 21(17):6072
Wojtczyk-Miaskowska A, Presler M, Michajlowski J et al (2017) Gene expression DNA methylation and prognostic significance of DNA repair genes in human bladder cancer. Cell Physiol Biochem 42(6):2404–2417
Friedrich MG, Chandrasoma S, Siegmund KD et al (2005) Prognostic relevance of methylation markers in patients with non-muscle invasive bladder carcinoma. Int J Cancer 41(17):2769–2778
Seah KS, Loha JY, TrangNguyen TT et al (2018) SAHA and cisplatin sensitize gastric cancer cells to doxorubicin by induction of DNA damage, apoptosis and perturbation of AMPK-mTOR signalling. Exp Cell Res 370(2):283–291
Galluzzi L, Senovilla L, Vitale I et al (2012) Molecular mechanisms of cisplatin resistance. Oncogene 31:1869–1883
Montazeri V, Ghahremani MH, Montazeri H et al (2020) A preliminary study of NER and MMR pathways involved in chemotherapy response in bladder transitional cell carcinoma: impact on progression-free survival. Iran J Pharm Res 19(1):355–365
Burgos-Molina AM, Mercado-Sáenz S, Cárdenas C et al (2021) Identification of new proteins related with cisplatin resistance in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 105:1965–1977
Stark AM, Doukas A, Hugo HH et al (2015) Expression of DNA mismatch repair proteins MLH1, MSH2, and MSH6 in recurrent glioblastoma. Neuro Res 37(2):1–11
Funding
This work was supported by University Grants Commission-National Eligibility Test Fellowship grant [Sr. No. 2061430780, Ref No.: 22/06/2014(i)EU-V to M.B.], Department of Biotechnology- West Bengal [923/(Sanc.)-BT (Estt.) RD 13/13F to C.K.P. and A.G.], Department of Science and Technology (Govt. of India), Kiran Division (SR/WOS-A/LS-278/2016 to D.M.) and NASI Senior Scientist Platinum Jubilee Fellowship (2020) to C.K.P.
Author information
Authors and Affiliations
Contributions
CKP, AG, DKP designed the study and brought funding from Department of Biotechnology, West Bengal. MB performed the experiments in the primary tumor samples and cell line. DKP provided the tumor samples and confirmed the histological stages of the tumor samples. MB and SG analyzed the results and did the statistical analysis. MB and CKP drafted the manuscript. DM and BC helped in the revision of the manuscript. The whole study was supervised by CKP and AG.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest.
Ethical approval
The study was done with appropriate approval of the Institutional Ethical Committee and have been performed in accordance with the ethical standards as laid down in the 1964 (Declaration of Helsinki).
Consent to participate
The study was done with informed consent from the patients and have been performed in accordance with the ethical standards as laid down in the 1964 (Declaration of Helsinki).
Consent for publication
A proper written consent for publication has been taken from either the patient or their guardian.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1007/s11010-024-05021-0
Supplementary Information
Below is the link to the electronic supplementary material.
11010_2022_4616_MOESM4_ESM.tif
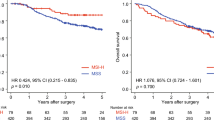
Supplementary file4 (TIF 449 KB) Fig. S1 Kaplan-Meier survival curve in patients with a MLH1 deletion b MSH2 deletion c MLH1 methylation
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Basu, M., Mukhopadhyay, D., Chakraborty, B. et al. RETRACTED ARTICLE: Differential operation of MLH1/MSH2 and FANCD2 crosstalk in chemotolerant bladder carcinoma: a clinical and therapeutic intervening study. Mol Cell Biochem 478, 1599–1610 (2023). https://doi.org/10.1007/s11010-022-04616-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04616-9