Abstract
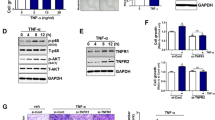
In cancer, the Epithelial to Mesenchymal Transition (EMT) is the process in which epithelial cells acquire mesenchymal features that allow metastasis, and chemotherapy resistance. Growth hormone (GH) has been associated with melanoma, breast, and endometrial cancer progression through an autocrine regulation of EMT. Since exogenous and autocrine expression of GH is known to have different molecular effects, we investigated whether exogenous GH is capable of regulating the EMT of cancer cells. Furthermore, we investigated whether exogenous GH could promote EMT in non-cancerous cells. To study the effect of GH (100 ng/ml) on cancer and non-cancer cells, we used HeLa and HEK293 cell lines, respectively. We evaluated the loss of cell–cell contacts, by cell scattering assay and migration by wound-healing assay. Additionally, we evaluated the morphological changes by phalloidin-staining. Finally, we evaluated the molecular markers E-cadherin and vimentin by flow cytometry. GH enhances cell scattering and the migratory rate and promotes morphological changes such as cell area increase and actin cytoskeleton filaments formation on HeLa cell line. Moreover, we found that GH favors the expression of the mesenchymal protein vimentin, followed by an increase in E-cadherin's epithelial protein expression, characteristics of an epithelial-mesenchymal hybrid phenotype that is associated with metastasis. On HEK293cells, GH promotes morphological changes, including cell area increment and filopodia formation, but not affects scattering, migration, nor EMT markers expression. Our results suggest that exogenous GH might participate in cervical cancer progression favoring a hybrid EMT phenotype but not on non-cancerous HEK293 cells.





Similar content being viewed by others
References
Diepenbruck M, Christofori G (2016) Epithelial–mesenchymal transition (EMT) and metastasis: yes, no, maybe? Curr Opin Cell Biol 43:7–13. https://doi.org/10.1016/j.ceb.2016.06.002
Nieto MA, Huang RYJ, Jackson RA, Thiery JP (2016) EMT: 2016. Cell 166(1):21–45. https://doi.org/10.1016/j.cell.2016.06.028
Pastushenko I, Blanpain C (2019) EMT transition states during tumor progression and metastasis. Trends Cell Biol 29(3):212–226. https://doi.org/10.1016/j.tcb.2018.12.001
Lamouille S, Xu J, Derynck R (2014) Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol 15(3):178–196. https://doi.org/10.1038/nrm3758
Ye X, Weinberg RA (2015) Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol 25(11):675–686. https://doi.org/10.1016/j.tcb.2015.07.012
Brittain AL, Basu R, Qian Y, Kopchick JJ (2017) Growth Hormone and the Epithelial-to-Mesenchymal Transition. J Clin Endocrinol Metab 102(10):3662–3673. https://doi.org/10.1210/jc.2017-01000
Zhu T, Starling-Emerald B, Zhang X, Lee KO, Gluckman PD, Mertani HC, Lobie PE (2005) Oncogenic transformation of human mammary epithelial cells by autocrine human growth hormone. Cancer Res 65(1):8. https://doi.org/10.1158/0008-5472.317.65.1
Nakonechnaya AO, Jefferson HS, Chen X, Shewchuk BM (2013) Differential effects of exogenous and autocrine growth hormone on LNCaP prostate cancer cell proliferation and survival. J Cell Biochem 114(6):1322–1335. https://doi.org/10.1002/jcb.24473
Gan Y, Shi C, Inge L, Hibner M, Balducci J, Huang Y (2010) Differential roles of ERK and Akt pathways in regulation of EGFR-mediated signaling and motility in prostate cancer cells. Oncogene 29(35):4947–4958. https://doi.org/10.1038/onc.2010.240
Al Moustafa AE, Achkhar A, Yasmeen A (2012) EGF-receptor signaling and epithelial-mesenchymal transition in human carcinomas. Front Biosci S4(2):671–684. https://doi.org/10.2741/s292
Di J, Huang H, Qu D, Tang J, Cao W, Lu Z, Cheng Q, Yang J, Bai J, Zhang Y, Zheng J (2015) Rap2B promotes proliferation, migration and invasion of human breast cancer through calcium-related ERK1/2 signaling pathway. Sci Rep 5(1):12363. https://doi.org/10.1038/srep12363
Fram ST, Wells CM, Jones GE (2011) HGF-induced DU145 cell scatter assay. In: Wells CM, Parsons M (eds) Cell migration, vol 769. Humana Press, pp 31–40
Lai JK, Wu HC, Shen YC, Hsieh HY, Yang SY, Chang CC (2012) Krüppel-like factor 4 is involved in cell scattering induced by hepatocyte growth factor. J Cell Sci. https://doi.org/10.1242/jcs.108910
Sridaran D, Ramamoorthi G, MahaboobKhan R, Kumpati P (2016) Oxystressed tumor microenvironment potentiates epithelial to mesenchymal transition and alters cellular bioenergetics towards cancer progression. Tumor Biol 37(10):13307–13322. https://doi.org/10.1007/s13277-016-5224-6
Devaraj V, Bose B (2019) Morphological state transition dynamics in EGF-induced epithelial to mesenchymal transition. J Clin Med 8(7):911. https://doi.org/10.3390/jcm8070911
von Laue S, Finidori J, Maamra M, Shen X, Justice S, Dobson P, Ross R (2000) Stimulation of endogenous GH and interleukin-6 receptors selectively activates different Jaks and Stats, with a Stat5 specific synergistic effect of dexamethasone. J Endocrinol 165(2):301–311. https://doi.org/10.1677/joe.0.1650301
Salt MB, Bandyopadhyay S, McCormick F (2014) Epithelial-to-mesenchymal transition rewires the molecular path to PI3K-dependent proliferation. Cancer Discov 4(2):186–199. https://doi.org/10.1158/2159-8290.CD-13-0520
van den Eijnden MJ, Strous GJ (2007) Autocrine growth hormone: effects on growth hormone receptor trafficking and signaling. Mol Endocrinol 21(11):2832–2846. https://doi.org/10.1210/me.2007-0092
Swerdlow A, Higgins C, Adlard P, Preece M (2002) Risk of cancer in patients treated with human pituitary growth hormone in the UK, 1959–85: a cohort study. Lancet 360(9329):273–277. https://doi.org/10.1016/S0140-6736(02)09519-3
Deodati A, Ferroli BB, Cianfarani S (2014) Association between growth hormone therapy and mortality, cancer and cardiovascular risk: Systematic review and meta-analysis. Growth Horm IGF Res 24(4):105–111. https://doi.org/10.1016/j.ghir.2014.02.001
Boguszewski CL, da Boguszewski MCS (2019) Growth hormone’s links to cancer. Endocr Rev 40(2):558–574. https://doi.org/10.1210/er.2018-00166
Allen DB, Backeljauw P, Bidlingmaier M, Biller BMK, Boguszewski M, Burman P, Butler G, Chihara K, Christiansen J, Cianfarani S, Clayton P, Clemmons D, Cohen P, Darendeliler F, Deal C, Dunger D, Erfurth EM, Fuqua JS, Grimberg A et al (2016) GH safety workshop position paper: a critical appraisal of recombinant human GH therapy in children and adults. Eur J Endocrinol 174(2):P1–P9. https://doi.org/10.1530/EJE-15-0873
Stepanenko AA, Dmitrenko VV (2015) HEK293 in cell biology and cancer research: Phenotype, karyotype, tumorigenicity, and stress-induced genome-phenotype evolution. Gene 569(2):182–190. https://doi.org/10.1016/j.gene.2015.05.065
Fischer V, Wong M, Li FQ, Takemaru KI (2017) Chibby1 knockdown promotes mesenchymal-to-epithelial transition-like changes. Cell Cycle 16(5):448–456. https://doi.org/10.1080/15384101.2017.1281478
Olarescu NC, Gunawardane K, Hansen TK, Møller N, Jørgensen JOL (2000) Normal physiology of growth hormone in adults. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA et al (eds) Endotext. MDTextcom Inc
Wideman L, Weltman JY, Hartman ML, Veldhuis JD, Weltman A (2002) Growth hormone release during acute and chronic aerobic and resistance exercise: recent findings. Sports Med 32(15):987–1004. https://doi.org/10.2165/00007256-200232150-00003
Godfrey RJ, Madgwick Z, Whyte G (2003) The exercise-induced growth hormone response in athletes. Sports Med 33:599–613. https://doi.org/10.2165/00007256-200333080-00005
Keller A, Wu Z, Kratzsch J, Keller E, Blum WF, Kniess A, Preiss R, Teichert J, Strasburger CJ, Bidlingmaier M (2007) Pharmacokinetics and pharmacodynamics of GH: dependence on route and dosage of administration. Eur J Endocrinol 156(6):647–653. https://doi.org/10.1530/EJE-07-0057
Langbakke IH, Nielsen JN, Skettrup MP, Harper A, Klitgaard T, Weil A, Engelhardt E, Lange M (2007) Pharmacokinetics and pharmacodynamics of growth hormone in patients on chronic haemodialysis compared with matched healthy subjects: an open, nonrandomized, parallel-group trial. Clin Endocrinol 67(5):776–783. https://doi.org/10.1111/j.1365-2265.2007.02962.x
Hou L, Chen Z, Liu D, Cheng Y, Luo X (2015) Comparative pharmacokinetics and pharmacodynamics of a PEGylated recombinant human growth hormone and daily recombinant human growth hormone in growth hormone-deficient children. Drug Des Devel Ther 10:13–21. https://doi.org/10.2147/DDDT.S93183
Smaniotto S, Mendes-da-Cruz DA, Carvalho-Pinto CE, Araujo LM, Dardenne M, Savino W (2010) Combined role of extracellular matrix and chemokines on peripheral lymphocyte migration in growth hormone transgenic mice. Brain Behav Immun 24(3):451–461. https://doi.org/10.1016/j.bbi.2009.11.014
Savino W, Smaniotto S, Mendes-da-Cruz DA, Dardenne M (2012) Growth hormone modulates migration of thymocytes and peripheral T cells: growth hormone modulates T cell migration. Ann NY Acad Sci 1261(1):49–54. https://doi.org/10.1111/j.1749-6632.2012.06637.x
Su HW, Lanning NJ, Morris DL, Argetsinger LS, Lumeng CN, Carter-Su C (2013) Phosphorylation of the adaptor protein SH2B1β regulates its ability to enhance growth hormone-dependent macrophage motility. J Cell Sci 126(8):1733–1743. https://doi.org/10.1242/jcs.113050
dos Santos Reis MD, dos Santos YMO, de Menezes CA, Borbely KSC, Smaniotto S (2018) Resident murine macrophage migration and phagocytosis are modulated by growth hormone: GH stimulates macrophage function. Cell Biol Int 42(5):615–623. https://doi.org/10.1002/cbin.10939
Ding J, Wirostko B, Sullivan DA (2015) Human growth hormone promotes corneal epithelial cell migration in vitro. Cornea 34(6):686–692. https://doi.org/10.1097/ICO.0000000000000418
Nakonechnaya AO, Shewchuk BM (2015) Growth hormone enhances LNCaP prostate cancer cell motility. Endocr Res 40(2):97–105. https://doi.org/10.3109/07435800.2014.966383
Mukhina S, Mertani HC, Guo K, Lee KO, Gluckman PD, Lobie PE (2004) Phenotypic conversion of human mammary carcinoma cells by autocrine human growth hormone. Proc Natl Acad Sci USA 101(42):15166–15171. https://doi.org/10.1073/pnas.0405881101
Shen C, Gu M, Song C, Miao L, Hu L, Liang D, Zheng C (2008) The tumorigenicity diversification in human embryonic kidney 293 cell line cultured in vitro. Biologicals 36(4):263–268. https://doi.org/10.1016/j.biologicals.2008.02.002
Goh ELK, Pircher TJ, Wood TJJ, Norstedt G, Graichen R, Lobie PE (1997) Growth hormone-induced reorganization of the actin cytoskeleton is not required for STAT5 (signal transducer and activator of transcription-5)-mediated transcription. Endocrinology 138(8):9. https://doi.org/10.1210/endo.138.8.5298
Herrington J, Diakonova M, Rui L, Gunter DR, Carter-Su C (2000) SH2-B is required for growth hormone-induced actin reorganization. J Biol Chem 275(17):13126–13133. https://doi.org/10.1074/jbc.275.17.13126
Diakonova M, Gunter DR, Herrington J, Carter-Su C (2002) SH2-Bβ Is a Rac-binding protein that regulates cell motility. J Biol Chem 277(12):10669–10677. https://doi.org/10.1074/jbc.M111138200
Lanning NJ, Carter-Su C (2007) Recent advances in growth hormone signaling. Rev Endocr Metab Disord 7(4):225–235. https://doi.org/10.1007/s11154-007-9025-5
Maures TJ, Kurzer JH, Carter-Su C (2007) SH2B1 (SH2-B) and JAK2: a multifunctional adaptor protein and kinase made for each other. Trends Endocrinol Metab 18(1):38–45. https://doi.org/10.1016/j.tem.2006.11.007
Rider L, Tao J, Snyder S, Brinley B, Lu J, Diakonova M (2009) Adapter protein SH2B1β cross-links actin filaments and regulates actin cytoskeleton. Mol Endocrinol 23(7):1065–1076. https://doi.org/10.1210/me.2008-0428
Kaulsay KK, Mertani HC, Lee KO, Lobie PE (2000) Autocrine human growth hormone enhancement of human mammary carcinoma cell spreading is Jak2 dependent. Endocrinology 141(4):14. https://doi.org/10.1210/endo.141.4.7426
Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D, Moers V, Lemaire S, De Clercq S, Minguijón E, Balsat C, Sokolow Y, Dubois C, De Cock F, Scozzaro S, Sopena F, Lanas A… Blanpain C, (2018) Identification of the tumour transition states occurring during EMT. Nature 556(7702):463–468. https://doi.org/10.1038/s41586-018-0040-3
Sulaiman A, Yao Z, Wang L (2018) Re-evaluating the role of epithelial-mesenchymal-transition in cancer progression. J Biomed Res 32(2):81–90. https://doi.org/10.7555/JBR.31.20160124
Kröger C, Afeyan A, Mraz J, Eaton EN, Reinhardt F, Khodor YL, Thiru P, Bierie B, Ye X, Burge CB, Weinberg RA (2019) Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc Natl Acad Sci USA 116(15):7353–7362. https://doi.org/10.1073/pnas.1812876116
Chao Y, Wu Q, Shepard C, Wells A (2012) Hepatocyte induced re-expression of E-cadherin in breast and prostate cancer cells increases chemoresistance. Clin Exp Metastasis 29(1):39–50. https://doi.org/10.1007/s10585-011-9427-3
Padmanaban V, Krol I, Suhail Y, Szczerba BM, Aceto N, Bader JS, Ewald AJ (2019) E-cadherin is required for metastasis in multiple models of breast cancer. Nature 573(7774):439–444. https://doi.org/10.1038/s41586-019-1526-3
Aceto N, Toner M, Maheswaran S, Haber DA (2015) In route to metastasis: circulating tumor cell clusters and epithelial-to-mesenchymal transition. Trends Cancer 1(1):44–52. https://doi.org/10.1016/j.trecan.2015.07.006
Acknowledgements
The authors thank Jonathan García Román, PhD., for his kind review of the final version of this manuscript. In addition, Olascoaga-Caso acknowledges the support from Consejo Nacional de Ciencia y Tecnología (CONACyT) for the doctoral scholarship assigned with the number 297526.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and experimental design. Data collection was performed by Olascoaga-Caso. Experimental design and analysis of scattering and migration assays were performed by Juárez-Aguilar and Olascoaga-Caso. Experimental design and analysis of cell morphology were performed by Tamariz-Domínguez and Olascoaga-Caso. Experimental design and analysis of flow cytometry were performed by Rodríguez-Alba and Olascoaga-Caso. The first draft of the manuscript was written by Olascoaga-Caso, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Olascoaga-Caso, E.M., Tamariz-Domínguez, E., Rodríguez-Alba, J.C. et al. Exogenous growth hormone promotes an epithelial-mesenchymal hybrid phenotype in cancerous HeLa cells but not in non-cancerous HEK293 cells. Mol Cell Biochem 478, 1117–1128 (2023). https://doi.org/10.1007/s11010-022-04583-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04583-1