Abstract
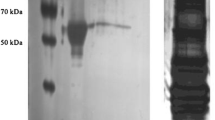
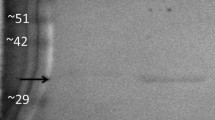
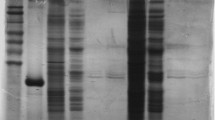
NADP-dependent isocitrate dehydrogenase (NADP-IDH, EC 1.1.1.42) catalyzes the oxidative decarboxylation of isocitrate to α-ketoglutarate with the concomitant production of NADPH. NADPH plays important roles in many biosynthesis pathways, maintenance of proper oxidation–reduction balance, and protection against oxidative damage. This present study investigated the dynamic nature of NADP-IDH during hibernation by purifying it from the skeletal muscle of Richardson's ground squirrel (Urocitellus richardsonii) and analyzing its structural and functional changes in response to hibernation. Kinetic parameters of purified NADP-IDH from euthermic and hibernating ground squirrel skeletal muscle were characterized at 22 °C and 5 °C. Relative to euthermic muscle, -NADP-IDH in hibernating muscle had a higher affinity for its substrate, isocitrate at 22 °C, whereas at 5 °C, there was a significant decrease in isocitrate affinity. Western blot analysis revealed greater serine and threonine phosphorylation in hibernator NADP-IDH as compared to euthermic NADP-IDH. In addition, Bioinformatic analysis predicted the presence of 18 threonine and 21 serine phosphorylation sites on squirrel NADP-IDH. The structural and functional changes in NADP-IDH indicate the ability of the organism to reduce energy consumption during hibernation, while emphasizing increased NADPH production, and thus antioxidant activity, during torpor arousal cycles.





Similar content being viewed by others
Data availability
The data are available on reasonable request.
References
Lyman CP, Willis JS, Malan A, Wang LCH (1984) Hibernation and torpor in mammals and birds. Academic Press, New York
Abnous K, Dieni CA, Storey KB (2008) Regulation of Akt during hibernation in Richardson’s ground squirrels. Biochim Biophys Acta—Gen Subj 1780:185–193. https://doi.org/10.1016/j.bbagen.2007.10.009
Ruberto AA, Childers CL, Storey KB (2016) Purification and properties of glycerol-3-phosphate dehydrogenase from the liver of the hibernating ground squirrel, Urocitellus richardsonii. Comp Biochem Physiol Part B Biochem Mol Biol 202:48–55. https://doi.org/10.1016/j.cbpb.2016.08.001
Wang LCH (1979) Time patterns and metabolic rates of natural torpor in the Richardson’s ground squirrel. Can J Zool 57:149–155. https://doi.org/10.1139/z79-012
Landau BR, Dawe AR (1958) Respiration in the hibernation of the 13-lined ground squirrel. Am J Physiol Content 194:75–82. https://doi.org/10.1152/ajplegacy.1958.194.1.75
Buck CL, Barnes BM (2000) Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol Regul Integr Comp Physiol 279:R255–R262. https://doi.org/10.1152/ajpregu.2000.279.1.R255
Wang LCH, Lee TF (1996) Torpor and hibernation in mammals: metabolic, physiological, and biochemical adaptations. Comprehensive Physiology. Wiley, Hoboken, pp 507–532
Storey KB (1997) Metabolic regulation in mammalian hibernation: enzyme and protein adaptations. Comp Biochem Physiol A Physiol 118:1115–1124. https://doi.org/10.1016/s0300-9629(97)00238-7
Carey HV, Andrews MT, Martin SL (2003) Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83:1153–1181. https://doi.org/10.1152/physrev.00008.2003
Storey KB, Storey JM (2004) Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev Camb Philos Soc 79:207–233. https://doi.org/10.1017/s1464793103006195
Brooks SP, Storey KB (1992) Mechanisms of glycolytic control during hibernation in the ground squirrel Spermophilus lateralis. J Comp Physiol B 162:23–28. https://doi.org/10.1007/BF00257932
MacDonald JA, Storey KB (1998) cAMP-dependent protein kinase from brown adipose tissue: temperature effects on kinetic properties and enzyme role in hibernating ground squirrels. J Comp Physiol B 168:513–525. https://doi.org/10.1007/s003600050172
Xu X, Zhao J, Xu Z et al (2004) Structures of human cytosolic NADP-dependent isocitrate dehydrogenase reveal a novel self-regulatory mechanism of activity. J Biol Chem 279:33946–33957. https://doi.org/10.1074/jbc.M404298200
Ju H-Q, Lin J-F, Tian T et al (2020) NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications. Signal Transduct Target Ther 5:231. https://doi.org/10.1038/s41392-020-00326-0
Thatcher BJ, Storey KB (2001) Glutamate dehydrogenase from liver of euthermic and hibernating Richardson’s ground squirrels: evidence for two distinct enzyme forms. Biochem Cell Biol 79:11–19. https://doi.org/10.1139/o00-086
Bell RAV, Dawson NJ, Storey KB (2012) Insights into the in vivo regulation of glutamate dehydrogenase from the foot muscle of an estivating land snail. Enzyme Res 2012:1–10. https://doi.org/10.1155/2012/317314
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brooks SPJ (1992) A simple computer program with statistical tests for the analysis of enzyme kinetics. Biotechniques 13(6):906–911
Blom N, Gammeltoft S, Brunak S (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294:1351–1362. https://doi.org/10.1006/jmbi.1999.3310
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201. https://doi.org/10.1093/bioinformatics/bti770
Bordoli L, Kiefer F, Arnold K et al (2009) Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc 4:1–13. https://doi.org/10.1038/nprot.2008.197
Biasini M, Bienert S, Waterhouse A et al (2014) SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res 42:W252–W258. https://doi.org/10.1093/nar/gku340
Brooks SPJ (1994) A program for analyzing enzyme rate data obtained from a microplate reader. Biotechniques 17(6):1154–1161
Zhang J, Storey KB (2016) RBioplot: an easy-to-use R pipeline for automated statistical analysis and data visualization in molecular biology and biochemistry. PeerJ 4:e2436. https://doi.org/10.7717/peerj.2436
Storey KB (2003) Mammalian hibernation. Transcriptional and translational controls. Adv Exp Med Biol 543:21–38
Ruf T, Geiser F (2015) Daily torpor and hibernation in birds and mammals. Biol Rev 90:891–926. https://doi.org/10.1111/brv.12137
Orr AL, Lohse LA, Drew KL, Hermes-Lima M (2009) Physiological oxidative stress after arousal from hibernation in arctic ground squirrel. Comp Biochem Physiol A Mol Integr Physiol 153:213–221. https://doi.org/10.1016/j.cbpa.2009.02.016
Storey KB, Storey JM (2012) Aestivation: signaling and hypometabolism. J Exp Biol 215:1425–1433. https://doi.org/10.1242/jeb.054403
Krivoruchko A, Storey KB (2015) Turtle anoxia tolerance: biochemistry and gene regulation. Biochim Biophys Acta—Gen Subj 1850:1188–1196. https://doi.org/10.1016/j.bbagen.2015.02.001
Moreira DC, Venancio LPR, Sabino MACT, Hermes-Lima M (2016) How widespread is preparation for oxidative stress in the animal kingdom? Comp Biochem Physiol Part A Mol Integr Physiol 200:64–78. https://doi.org/10.1016/j.cbpa.2016.01.023
Tessier SN, Breedon SA, Storey KB (2021) Modulating Nrf2 transcription factor activity: revealing the regulatory mechanisms of antioxidant defenses during hibernation in 13-lined ground squirrels. Cell Biochem Funct 39:623–635. https://doi.org/10.1002/cbf.3627
Zera AJ, Newman S, Berkheim D et al (2011) Purification and characterization of cytoplasmic NADP+-isocitrate dehydrogenase, and amplification of the NADP+-IDH gene from the wing-dimorphic sand field cricket Gryllus firmus. J Insect Sci 11:53. https://doi.org/10.1673/031.011.5301
Vucetic M, Stancic A, Otasevic V et al (2013) The impact of cold acclimation and hibernation on antioxidant defenses in the ground squirrel (Spermophilus citellus). Free Radic Biol Med 65:916–924. https://doi.org/10.1016/j.freeradbiomed.2013.08.188
Allan ME, Storey KB (2012) Expression of NF-κB and downstream antioxidant genes in skeletal muscle of hibernating ground squirrels, Spermophilus tridecemlineatus. Cell Biochem Funct 30:166–174. https://doi.org/10.1002/cbf.1832
Morin P, Storey KB (2006) Evidence for a reduced transcriptional state during hibernation in ground squirrels. Cryobiology 53:310–318. https://doi.org/10.1016/j.cryobiol.2006.08.002
Frerichs KU, Smith CB, Brenner M et al (1998) Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci 95:14511–14516. https://doi.org/10.1073/pnas.95.24.14511
Cai D, McCarron RM, Yu EZ et al (2004) Akt phosphorylation and kinase activity are down-regulated during hibernation in the 13-lined ground squirrel. Brain Res 1014:14–21. https://doi.org/10.1016/j.brainres.2004.04.008
Storey KB (2010) Out cold: biochemical regulation of mammalian hibernation—A mini-review. Gerontology 56:220–230. https://doi.org/10.1159/000228829
Biggar Y, Storey KB (2014) Global DNA modifications suppress transcription in brown adipose tissue during hibernation. Cryobiology 69:333–338. https://doi.org/10.1016/j.cryobiol.2014.08.008
Brown JCL, Chung DJ, Belgrave KR, Staples JF (2012) Mitochondrial metabolic suppression and reactive oxygen species production in liver and skeletal muscle of hibernating thirteen-lined ground squirrels. Am J Physiol Regul Integr Comp Physiol 302:R15-28. https://doi.org/10.1152/ajpregu.00230.2011
Abnous K, Storey KB (2007) Regulation of skeletal muscle creatine kinase from a hibernating mammal. Arch Biochem Biophys 467:10–19. https://doi.org/10.1016/j.abb.2007.07.025
Abnous K, Storey KB (2008) Skeletal muscle hexokinase: regulation in mammalian hibernation. Mol Cell Biochem 319:41–50. https://doi.org/10.1007/s11010-008-9875-5
Bell RAV, Storey KB (2018) Purification and characterization of skeletal muscle pyruvate kinase from the hibernating ground squirrel, Urocitellus richardsonii: potential regulation by posttranslational modification during torpor. Mol Cell Biochem 442:47–58. https://doi.org/10.1007/s11010-017-3192-9
Green SR, Storey KB (2021) Skeletal muscle of torpid Richardson’s ground squirrels (Urocitellus richardsonii) exhibits a less active form of citrate synthase associated with lowered lysine succinylation. Cryobiology 101:28–37. https://doi.org/10.1016/j.cryobiol.2021.06.006
Ramnanan CJ, McMullen DC, Bielecki A, Storey KB (2010) Regulation of sarcoendoplasmic reticulum Ca2+-ATPase (SERCA) in turtle muscle and liver during acute exposure to anoxia. J Exp Biol 213:17–25. https://doi.org/10.1242/jeb.036087
Ruberto AA, Logan SM, Storey KB (2019) Temperature and serine phosphorylation regulate glycerol-3-phosphate dehydrogenase in skeletal muscle of hibernating Richardson’s ground squirrels. Biochem Cell Biol 97:148–157. https://doi.org/10.1139/bcb-2018-0198
Storey KB, Storey JM (2005) Biochemical adaptation to extreme environments. Integrative Physiology in the Proteomics and Post-Genomics Age. Humana Press, Totowa, pp 169–200
Acknowledgements
The authors thank S.A. Breedon and C.L. Childers for their help and editorial review of this manuscript. The authors also thank J.M. Storey for editorial review of this manuscript.
Funding
The research was funded by a Discovery Grant (Number# 6793) through the Natural Sciences and Engineering Research Council of Canada (NSERC). K.B. Storey currently holds the Canada Research Chair in Molecular Physiology.
Author information
Authors and Affiliations
Contributions
IAM and KBS conceptualized and designed the project and IAM conducted all experiments. Data analysis and assembly of the manuscript were carried out by AV, IAM, and KBS.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All protocols for animal care and treatment had prior approval by the Carleton University Animal Care Committee and met guidelines for the Canadian Council on Animal Care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
MacLean, I.A., Varma, A. & Storey, K.B. Purification and characterization of NADP-isocitrate dehydrogenase from skeletal muscle of Urocitellus richardsonii. Mol Cell Biochem 478, 415–426 (2023). https://doi.org/10.1007/s11010-022-04516-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-022-04516-y